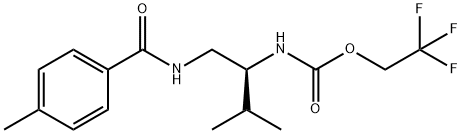
How is Tolprocarb synthesised?
Synthesis of Tolprocarb
The synthesis of Tolprocarb was initiated by the carbamate coupling of trifluoroethyl chloroformate and valine under basic biphasic reaction conditions. The specific synthesis steps are as follows:
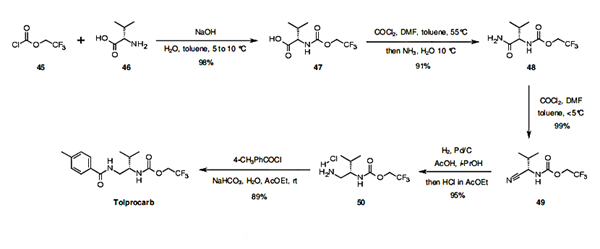
The first step of the synthesis of tolprocarb is the carbamate coupling of valine (46) with trifluoroethyl chloroformate (45) in basic biphasic reaction conditions. The carbamate 47 thus obtained is then treated sequentially with phosgene and ammonia to yield carboxamide 48, which is again reacted with phosgene to afford the nitrile intermediate 49. Palladium catalyzed hydrogenation of 49, followed by amidation of 50 with 4-methylbenzoic acid, yields tolprocarb in 6 synthetic steps and 75% overall yield.
Introduction of Tolprocarb
The valine carbamate fungicide tolprocarb was presented by Mitsui Chemicals in 2012. It is being developed with a focus on the rice market, where it shows good control of Magnaporthe grisea, the causal agent of rice blast. Tolprocarb's fungicidal activity results from the inhibition of melanin biosynthesis, an essential step which allows the fungal appressoria to infect host plants. While other agrochemicals are known to inhibit reductase and dehydratase enzymes in the melanin biosynthesis pathway, tolprocarb is unique in that it inhibits the polyketide synthase enzyme (PKS).