Is benzoic acid polar or nonpolar?
Benzoic acid is an aromatic carboxylic acid. The carboxylic acid (COOH) functional group is attached to the phenyl ring.
Is benzoic acid polar?
Benzoic acid is a polar molecule. The polarity of benzoic acid is accredited to the presence of a polar carboxyl (COOH) functional group in it. The C-O, C=O and O-H bonds present in the COOH group are strongly polar.
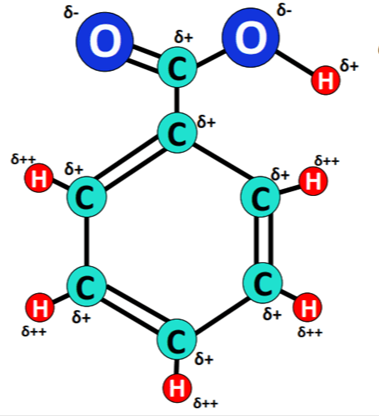
A high electronegativity difference of 0.89 units is present between a C-atom and an O-atom in the C-O or C=O bond.
Benzoic acid is considered polar due to the presence of the polar carboxylic acid group, which imparts a significant dipole moment to the molecule. Although the benzene ring is nonpolar, the overall molecule exhibits polarity because of the carboxylic acid group's influence.
Is benzoic acid soluble in water?
Benzene is the main ingredient in benzoic acid, the majority of benzoic acid is nonpolar, making the acid only slightly water soluble. However, its solubility increases as the temperature increases.
Benzoic acid is soluble in hot water. However, it is only sparingly soluble in cold water. Benzoic acid molecules can develop intermolecular H-bonding using their hydroxyl (OH) functional groups.
In summary, benzoic acid is a polar molecule because of a polar functional group (carboxylic acid) which dominates its interactions with other polar substances, such as water, despite having a nonpolar benzene ring as part of its structure.
You may like
Related articles And Qustion
See also
Lastest Price from Benzoic acid manufacturers

US $50.00-10.00/kg2025-09-02
- CAS:
- 65-85-0
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Single metal impurity≤ 100ppb) or pharmaceutical grade
- Supply Ability:
- 100kg

US $1.00/KG2025-06-27
- CAS:
- 65-85-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt