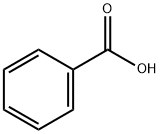
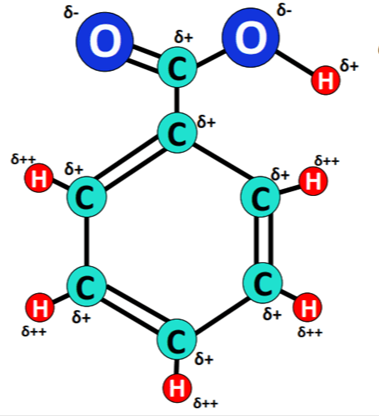
Preparation and Synthesis Method of Benzoic Acid
Benzoic acid is a colorless crystal solid that can reduce the energy of the surface in a reaction. This acid can be dissolved in water at less than 3 g per liter. A thin film of silver is created at room temperature on a clean copper substrate with simple soaking in blue silver nitrate solution with or without the presence of adequate concentrations of benzoic acid during a specified period of time. This compound belongs to the class of organic compounds known as benzoic acids. These are organic Compounds containing a benzene ring which bears at least one carboxyl group.
Application of Benzoic Acid
Benzoic acid (BA) is a traditional food preservative to prevent the growth of bacteria, yeasts, and molds. However, its excessive use could lead to metabolic acidosis, convulsions, and hyperpnoea in humans. Its antimicrobial activity in foods is due to the nonionized form of BA, which is the predominant form at pH values of around 3 or lower, and hence it is exceptionally suitable as preservatives for acid matrices such as fruit juice. Its mechanism is based on the absorption BA across the cell membrane where anaerobic fermentation of glucose via phosphofructokinase is reduced. The benefits of the use of BA are the low toxicity and the lack of color. However, the disadvantage is its limited solubility in aqueous solution. Its sodium or potassium salts are readily soluble where potassium salt is normally preferable in order to minimize the sodium level in a food product.
Synthesis of Benzoic Acid
Benzoic acid exists as a crystalline, colourless solid under normal conditions. The term ‘benzoate’ refers to the esters and salts of C6H5COOH.
The commercial production of benzoic acid is done via the partial oxidation of toluene with oxygen, catalyzed by manganese or cobalt naphthenates. This chemical reaction is illustrated below.
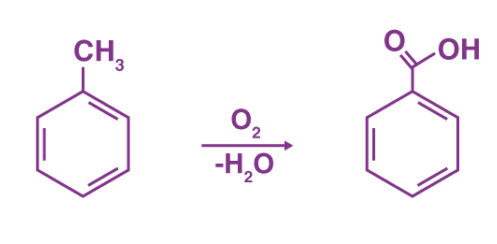
Another industrial method of preparing benzoic acid is by reacting tri-chlorotoluene with calcium hydroxide in the presence of water, and the treatment of the calcium benzoate product with hydrochloric acid.
Related articles And Qustion
Lastest Price from Benzoic acid manufacturers

US $50.00-10.00/kg2025-09-02
- CAS:
- 65-85-0
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Single metal impurity≤ 100ppb) or pharmaceutical grade
- Supply Ability:
- 100kg

US $1.00/KG2025-06-27
- CAS:
- 65-85-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt