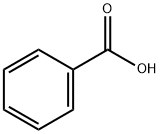
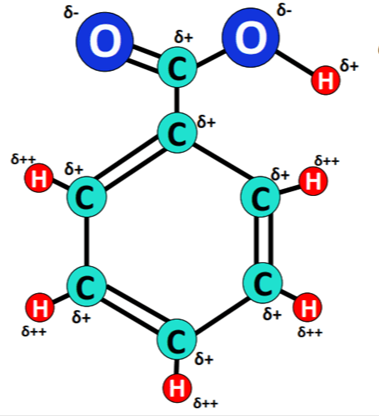
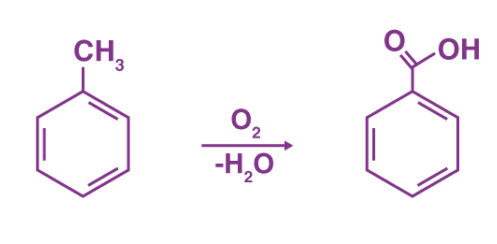Is benzoic acid solubility in water?
Benzoic acid is not very soluble in water. It has a high solubility in hot water while poor solubility in cold water. The solubility of Benzoic acid in water increases when the temperature is increased. At a temperature of 0°C, the solubility of benzoic acid in water corresponds to 1.7 grams per litre. When heated to 100 °C, the solubility of this compound in water increases to 56.31 grams per litre.

Also Benzoic acid can easily dissolve in organic solvents like acetone, benzene, alcohol, liquid ammonia, ether, CCl₄, etc. The solubility is high in ethanol, reasonably high in chloroform, lower in toluene, and quite low in the remaining three pure solvents.
In the binary mixtures the solubility of benzoic acid increases with increasing concentration of ethanol. The solubility of benzoic acid increases with increasing temperature.
You may like
Related articles And Qustion
See also
Lastest Price from Benzoic acid manufacturers

US $50.00-10.00/kg2025-09-02
- CAS:
- 65-85-0
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Single metal impurity≤ 100ppb) or pharmaceutical grade
- Supply Ability:
- 100kg

US $1.00/KG2025-06-27
- CAS:
- 65-85-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt