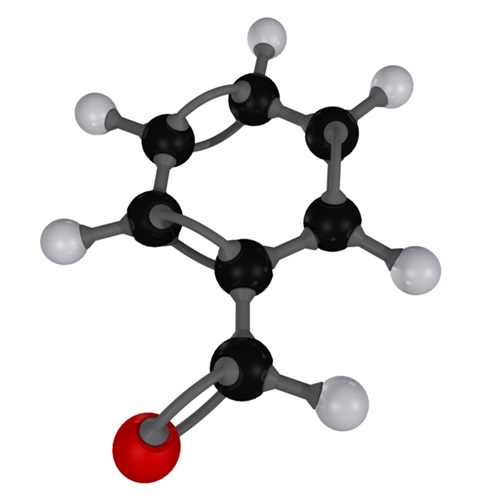
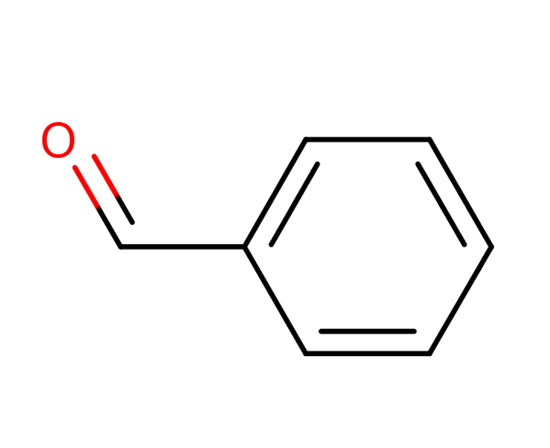
Exploration of Benzaldehyde: Synthesis, Properties, and Applications
Benzaldehyde, an aromatic aldehyde with the molecular formula C7H6O, is a significant compound in the chemical industry due to its versatile applications. It is known for its distinctive bitter almond smell and is found naturally in the glucoside amygdalin present in bitter almonds.
Synthesis of Benzaldehyde
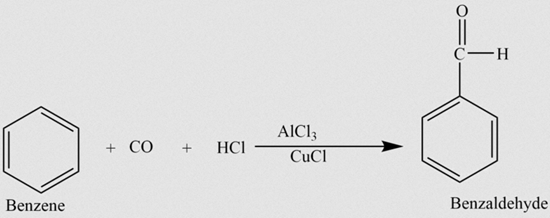
1. Gattermann-Koch Aldehyde Synthesis:
In this method, benzene reacts with carbon monoxide and hydrochloric acid in the presence of anhydrous aluminum chloride and a trace amount of copper (I) chloride. Copper (I) chloride acts as a co-catalyst in this reaction, which is named after its discoverers, Gattermann and Koch.
2. Gattermann Reaction:
This process involves the reaction of benzene with a mixture of hydrogen cyanide and hydrogen chloride, catalyzed by aluminum chloride, resulting in the formation of benzaldehyde.
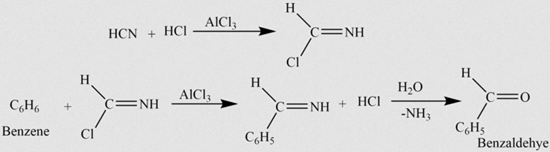
3. Grignard Reaction:
Phenyl magnesium bromide, a Grignard reagent, reacts with ethyl formate to produce benzaldehyde. This reaction is a classic example of the versatility of Grignard reagents in organic synthesis.
4. From Toluene:
Toluene can be converted to benzaldehyde through its reaction with chromyl chloride in a carbon tetrachloride solution, which forms an intermediate complex. This complex, upon decomposition with water, yields benzaldehyde.
5. Rosenmund’s Reaction:
This is a catalytic hydrogenation process where acetyl chloride is reduced in the presence of a palladium catalyst that has been poisoned with barium sulfate, resulting in the production of benzaldehyde.
6. From Diazonium Salt:
Benzene diazonium chloride reacts with formaldoxime to produce benzaldehyde. This method is an example of the use of diazonium salts in the synthesis of aromatic compounds.
Industrial Applications
Benzaldehyde's industrial applications are diverse, ranging from the preparation of dyes, cosmetic products, and flavoring agents to its use as a starting material in the synthesis of pharmaceuticals. Its ability to impart a distinct almond flavor makes it a common ingredient in food and beverage industries.
Benzaldehyde, with its wide range of synthesis methods and unique properties, plays a crucial role in the chemical industry. Its applications extend beyond the laboratory into everyday products, highlighting the importance of understanding and optimizing its production processes. As research continues, new methods of synthesis and applications for benzaldehyde are likely to emerge, further expanding its relevance in the chemical and allied industries.
You may like
Related articles And Qustion
See also
Lastest Price from Benzaldehyde manufacturers
US $0.00-0.00/kg2025-12-13
- CAS:
- 100-52-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $5.00-2.00/KG2025-06-03
- CAS:
- 100-52-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg