Polarity of Benzaldehyde
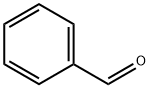
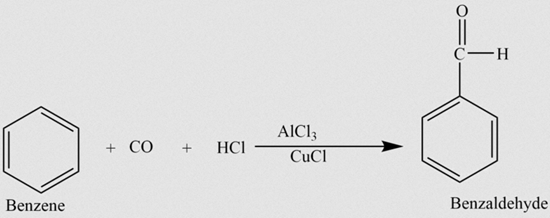
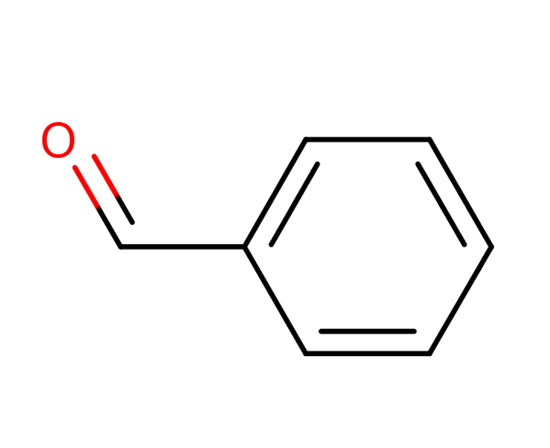
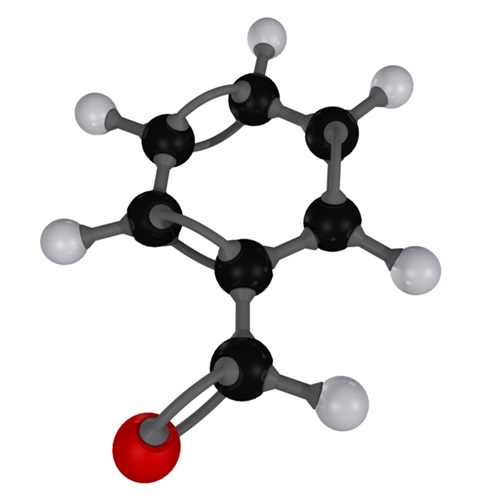
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
Uses
Benzaldehyde is a colorless liquid with a characteristic almond-like odor, and is commonly used in cherry-flavored sodas. A component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources. Synthetic benzaldehyde is the flavoring agent in imitation almond extract, which is used to flavor cakes and other baked goods.
Polarity or unpolar
The difference in electronegativity and electron sharing between two atoms in a covalent bond determines whether the molecule is polar or non-polar.
When the sharing of electrons between two atoms is unequal, the molecule is said to be polar, and when the sharing of electrons between the atoms is equal, the molecule is said to be non-polar.
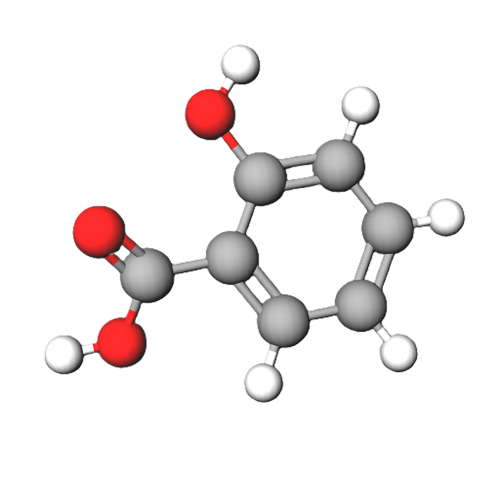
Benzaldehyde (C6H5CHO) is a polar compound. This is because of the unbalanced electron density. The electronegativity difference between hydrogen and carbon is negligible, whereas the electronegativity difference between carbon and oxygen is large enough to cause polarity.
This means that electrons in the double bond are more attracted to oxygen, causing charges to shift, resulting in a partial positive charge on the carbon and a partial negative charge on the oxygen.
The difference in electronegativity allows polarity to make a molecule non-symmetrical (asymmetrical), allowing the -ve charge to be centralised on one side of the molecule by the oxygen.
You may like
Related articles And Qustion
Lastest Price from Benzaldehyde manufacturers
US $0.00-0.00/kg2025-12-13
- CAS:
- 100-52-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $5.00-2.00/KG2025-06-03
- CAS:
- 100-52-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg