The Polar and Non-polar Characteristics in the Structure of Salicylic Acid
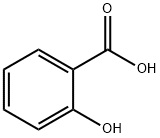
Salicylic acid is an organic compound, a colorless, bitter-tasting solid, it is a precursor to and a metabolite of aspirin (acetylsalicylic acid). This article will introduce its polarity.
An organic molecule can have polar and a non-polar regions. Whether the molecule behaves more like a polar or non-polar molecule will depend on which region is more significant.
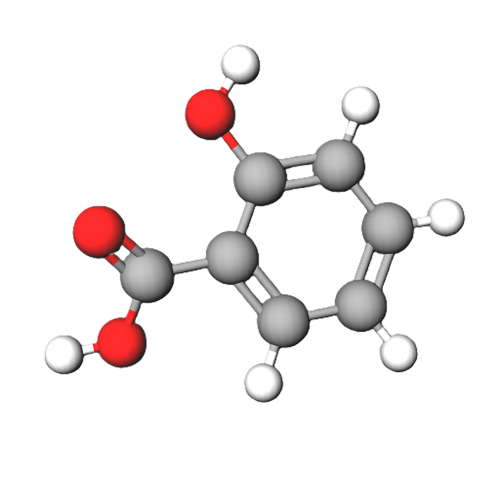
Salicylic acid has two polar regions, a carboxyl group (giving it it's acidic property) and a hydroxyl group. However, these two groups are attached to a benzene ring which is a non-polar 6 carbon hydrocarbon. This non-polar ring is rather large in comparison to the polar regions of the molecule and so will significantly influence it's properties.
The properties of salicylic acid reflect these polar/non-polar characteristics. The boiling point of salicylic acid (211 °C) is significantly higher than that of non-polar benzene (80 °C), we can attribute this to the stronger intermolecular forces that the polar regions allow salicylic acid to form (H-bonds + dipole-dipole forces).
Therefore, salicylic acid is slightly soluble in water. At room temperature, it can dissolve in water to a limited extent. However, it is more soluble in organic solvents such as ethanol and ether.
Related articles And Qustion
See also
Lastest Price from Salicylic acid manufacturers

US $0.00-0.00/KG2025-11-29
- CAS:
- 69-72-7
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00-0.00/kg2025-08-08
- CAS:
- 69-72-7
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 10000kg