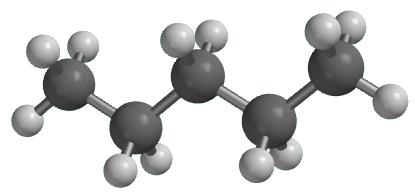
Why is Pentane nonpolar?
Pentane is a natural product found in Calendula officinalis and Allium ampeloprasum.It appears as a clear colorless liquid with a petroleum-like odor. Less dense than water and insoluble in water. Hence floats on water. Vapors are heavier than air.
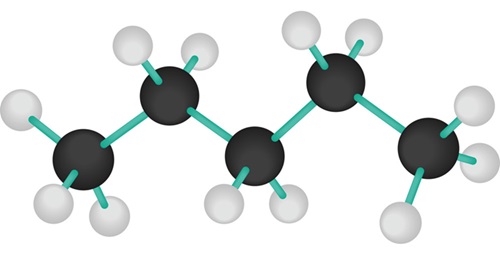
Polarity of Pentane
The difference in electronegativity and electron sharing between two atoms in a covalent bond determines whether the molecule is polar or non-polar.
When the sharing of electrons between two atoms is unequal, the molecule is said to be polar, and when the sharing of electrons between the atoms is equal, the molecule is said to be non-polar.
Pentane is considered to be nonpolar since the only two types of bonds,C-C and C-H,and nonpolar.The borderline between polar and nonpolar bonds is usually considered to be a 0.5 difference between the electronegativity of the two atoms that take part in them.
The difference between carbon(2.5)and hydrogen(2.1)is only of 0.4.
Related articles And Qustion
See also
Lastest Price from Pentane manufacturers
US $1.00/KG2025-04-21
- CAS:
- 109-66-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $1300.00/T2024-05-07
- CAS:
- 109-66-0
- Min. Order:
- 1T
- Purity:
- 98%
- Supply Ability:
- 20T