A small-molecule inhibitor: IPI 145(Duvelisib)
Description
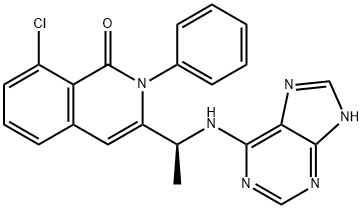
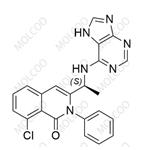
Duvelisib (IPI 145) is a small-molecule inhibitor of phosphatidylinositol-3 kinase that has been developed as an oral treatment for various cancer indications[1]. Its IUPAC name is (S)-3-(1-((7H-purin-6-yl)amino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one.
Biological action
IPI 145 is a dual PI3Kδ and PI3Kγ inhibitor with enhanced selectivity for PI3Kδ (10-fold) over PI3Kγ. The selectivity for PI3Kδ over PI3Kα can be attributed to the conformation that the compounds adopt in the active site, namely propeller-shaped, which opens a hydrophobic pocket that is not present in the apoenzyme[2]. It was shown to be selective for inhibiting PI3K from class I, not being active against other protein or lipid kinases.
After initial development by Intellikine and further work by Infinity Pharmaceuticals, duvelisib monohydrate was licensed to Verastem Oncology in 2016 for commercialization.
Synthetic method
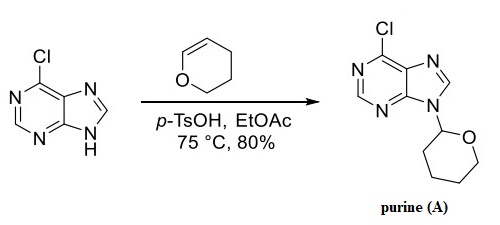
A kilogram-scale synthesis of duvelisib was disclosed in a 2011 patent application from Intellikine and relied upon the THP-protected purine (A), available in a single step from widely available 6-chloro-9H-purine and dihydropyran, shown above.
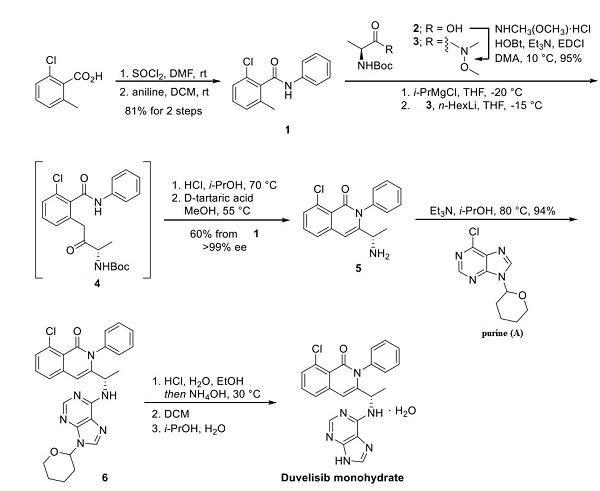
Preparation of the heterocyclic architecture of the drug began with 2-chloro-6-methylbenzoic acid (shown above), which was activated with thionyl chloride and treated with aniline to give benzamide 1. Compound 1 was treated with isopropylmagnesium chloride in chilled THF in an impressive telescoped sequence. In a separate reactor, Weinreb amide 3 (accessible from L-alanine (2)) was simultaneously treated with n-hexyl lithium. These separate solutions were combined to give rise to intermediate 4, which was then quenched with HCl, resulting in Boc deprotection and cyclization to give isoquinolone 5, albeit with an erosion of enantiopurity (84% ee). A salt resolution by treatment with D-tartaric acid in methanol, filtration, and treatment with ammonium hydroxide improved the enantiomeric excess of 5 to >99% while delivering an overall yield of 60% from compound 1. SNAr then installed the purine fragment with purine (A). Acidic THP removal, pH adjustment with ammonium hydroxide, and isolation from DCM preceded recrystallization in isopropanol and water to give duvelisib in the desired solid form after drying.
References
[1] Blair, Hannah A. “Duvelisib: First Global Approval.” Drugs (2018): 1847–1853.
[2] Daniel A Rodrigues, Carlos A M Fraga, Fernanda S Sagrillo. “Duvelisib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Phosphoinositide 3-Kinases.” Pharmaceuticals (2019).
[3] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
You may like
See also
Lastest Price from IPI 145 manufacturers

US $1.00/g2025-08-19
- CAS:
- 1201438-56-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg
US $0.00-0.00/mg2025-04-18
- CAS:
- 1201438-56-3
- Min. Order:
- 10mg
- Purity:
- 98
- Supply Ability:
- 10000000