Pentane - Uses, Mechanism of Toxicity, and Environmental Fate
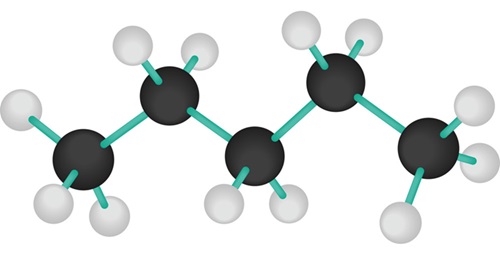
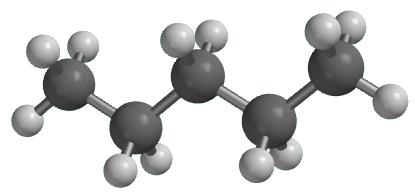
Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two are called isopentane (methylbutane) and neopentane (dimethylpropane). Cyclopentane is not an isomer of pentane because it has only 10 hydrogen atoms where pentane has 12.
Uses
Pentane is present in volatile petroleum fractions and is used as
a fuel; in the production of ammonia, olefin, and hydrogen; in
the manufacture of artificial ice; in low-temperature thermometers;
as a blowing agent for plastics and foams; and in
solvent extraction processes. Neopentane is important in the
manufacture of rubber.
Mechanism of Toxicity
The mechanism of toxicity is suspected to be similar to other
solvents that rapidly induce anesthesia-like effects, that is
a ‘nonspecific narcosis’ due to disruption (solvation) of the
integrity of the cellular membranes of the central nervous
system. The effect is similar to the ‘high’ experienced upon
exposure to other aliphatic hydrocarbon solvents.
As seen with other short-chain alkanes, upon inhalation,
pentane is moderately toxic and may cause irritation of the
respiratory tract and narcosis. The narcotic action of pentane
(observed following 1-h exposure to 90 000–120 000 ppm) is,
however, much less pronounced than effects seen following
exposure to the C1–C4 alkanes. Although the actual biochemical
mechanismof toxicity has not been discerned, the narcotic effects
seen are most likely related to its physical solvent properties.
The mechanism of toxicity is suspected to be similar to other
solvents that rapidly induce anesthesia-like effects, that is
a ‘nonspecific narcosis’ due to disruption (solvation) of the
integrity of the cellular membranes of the central nervous
system. The effect is similar to the ‘high’ experienced upon
exposure to other aliphatic hydrocarbon solvents.
As seen with other short-chain alkanes, upon inhalation, pentane is moderately toxic and may cause irritation of the respiratory tract and narcosis. The narcotic action of pentane (observed following 1-h exposure to 90 000–120 000 ppm) is, however, much less pronounced than effects seen following exposure to the C1–C4 alkanes. Although the actual biochemical mechanismof toxicity has not been discerned, the narcotic effects seen are most likely related to its physical solvent properties.
Environmental Fate
Pentane is a five-carbon aliphatic compound that is a natural constituent of the major paraffin fraction of crude oil and also found in natural gas. Pentane is a colorless, flammable liquid (the first liquid member of the alkanes) that is lighter than water. It has a pleasant odor with an olfactory threshold of 900 ppm, and a moderate odor intensity is observed at 5000 ppm. It occurs as two other isomers, including isopentane, (CH3)2CHCH2CH3, and neopentane, C(CH3)4. Isopentane (2-methylbutane) apparently has physical and physiological characteristics similar to straight-chain pentane. Neopentane (2,2-dimethylpropane) is similar to butane in physical and physiological characteristics. Pentane has a molecular weight of 72.15 g mol-1. At 25 °C, pentane has a solubility in water of 38 mg l-1, an estimated vapor pressure of 514 mmHg, and a Henry’s law constant of 1.25 atmm3 mol-1 (http://www.epa.gov/oppt/exposure/pubs/episuite .htm). The log octanol/water partition coefficient is 3.39. Conversion factors for pentane in air are as follows: 1 mgm-3¼ 0.34 ppm and 1 ppm ¼ 2.95 mgm-3.
If released to air, the relatively high vapor pressure indicates pentane will exist solely as a vapor in the ambient atmosphere. Vapor-phase pentane will be degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 4 days. Vapor-phase pentane will neither undergo hydrolysis in the environment due to the lack of hydrolyzable functional groups nor photolyze due to the lack of absorption in the ultraviolet spectrum (>290 nm) (http://www.toxnet .nlm.nih.gov).
If released to soil, n-pentane is expected to have high mobility based upon the relatively low Koc. Volatilization from moist soil surfaces is expected to be an important fate process based upon a Henry’s law constant of 1.25 atm-m3 mol-1. n-Pentane may volatilize from dry soil surfaces based upon its vapor pressure. Screening studies suggest that n-pentane will undergo biodegradation in soil and water surfaces, but volatilization is expected to be the predominant fate process in the environment (http://www.toxnet.nlm.nih.gov).
You may like
Related articles And Qustion
See also
Lastest Price from Pentane manufacturers
US $1.00/KG2025-04-21
- CAS:
- 109-66-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $1300.00/T2024-05-07
- CAS:
- 109-66-0
- Min. Order:
- 1T
- Purity:
- 98%
- Supply Ability:
- 20T