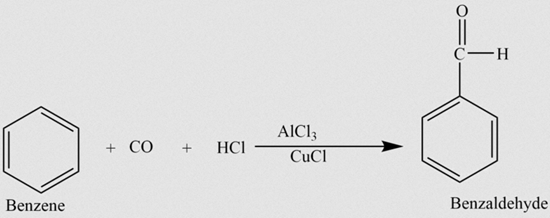
Structure, Intermolecular Interactions and Applications of Enzaldehyde
The benzaldehyde is an organic compound whose chemical formula is C6H5CHO. At room temperature, it is a colorless liquid that may turn yellowish on storage. Benzaldehyde represents the simplest aromatic aldehyde and the most used industrially.
Structure
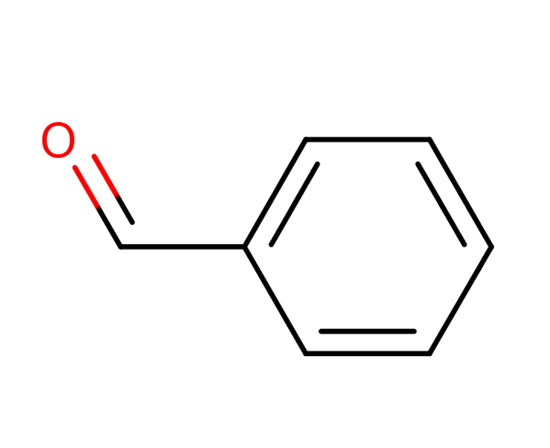
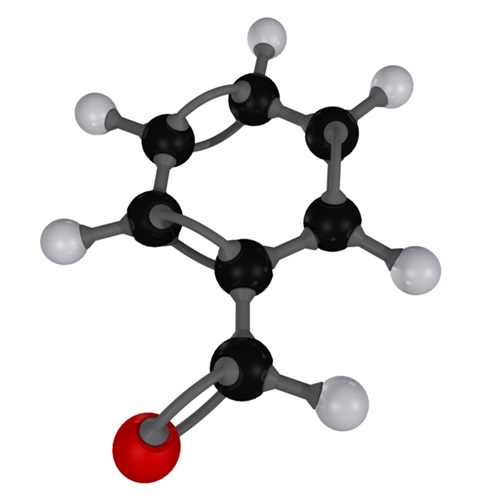
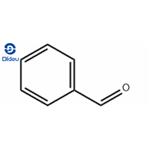
As can be seen in the above image, the structure of benzaldehyde reveals its aromatic character -the benzene ring on the left- and also the formyl group (-CHO), on the right, responsible for the polar character of the molecule. So, benzaldehyde is an organic, aromatic and polar compound.
Intermolecular Interactions
The formyl group establishes a permanent dipole moment in the benzaldehyde molecule, although remarkably weak compared to that of benzoic acid. This allows it to have stronger intermolecular interactions than benzene, whose molecules can only interact through London forces (induced dipole-dipole scattering).
This is reflected in its physical properties, such as the boiling point, which is twice that of benzene (80 ºC).
Also, the formyl group lacks the ability to form hydrogen bonds (hydrogen is bonded to carbon, not oxygen). This makes it impossible for benzaldehyde molecules to form three-dimensional arrangements, like those seen in benzoic acid crystals.
Applications
Benzaldehyde is a compound that serves as the basis for medicines, colorants, perfumes and in the resin industry. It can also be used as a solvent, plasticizer, and low-temperature lubricant. It is used to flavor or season food and tobacco.
You may like
Related articles And Qustion
See also
Lastest Price from Benzaldehyde manufacturers
US $0.00-0.00/kg2025-12-13
- CAS:
- 100-52-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $5.00-2.00/KG2025-06-03
- CAS:
- 100-52-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg