How to synthesize Dacomitinib (PF299804)?
Description
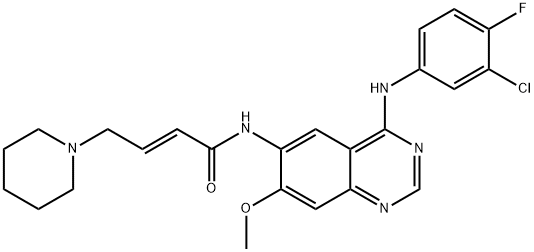
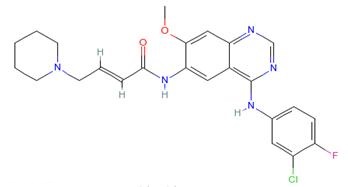
Dacomitinib (PF299804), developed by Pfizer, is a second-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), superior to first-generation EGFR TKI in ARCHER 1050. The drug also binds to EGFR/HER1, HER2, and HER4[1].
The drug was approved by the USFDA in September 2018 for first-line treatment of patients with metastatic non-small-cell lung cancer (NSCLC) with EGFR exon 19 deletion or exon 21 L858R substitution mutations, as detected by a USFDA-approved test. Second-generation EGFR TKIs, including dacomitinib, have been designed as pan-HER inhibitors to overcome issues of acquired resistance associated with first-generation EGFR TKIs, which include gefitinib and erlotinib. In a phase III clinical trial, dacomitinib prolonged progression-free survival compared to gefitinib. The primary literature has published accounts of the medicinal chemistry discovery and process development optimization of dacomitinib.
Synthetic method
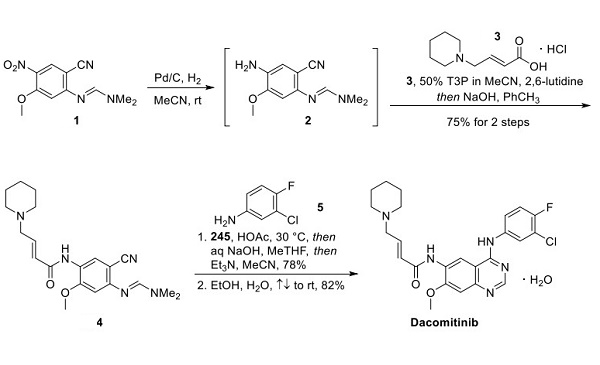
Scheme 1. Synthesis of Dacomitinib
The commercial process for synthesizing dacomitinib, as reported by authors from Pfizer and described above(Scheme 1), involves an efficient three-step, two-isolation sequence that delivered dacomitinib in an impressive 58% overall yield. Hydrogenative reduction of nitroarene 1 provided aniline 2 as a solution in acetonitrile, which was carried forward into the next step without purification. Amidation of aniline 2 with butenoic acid 3, synthesized in two steps from bromide 6 (Scheme 2), was facilitated by T3P and 2,6- lutidine in acetonitrile. A reverse quench with aqueous NaOH was subsequently performed to minimize hydrolysis of the formamidine group upon workup. A seeded, cooling crystallization from a mixture of acetonitrile and toluene then provided amide 4 in 75% over two steps.
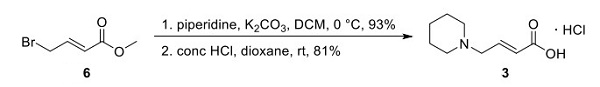
Scheme 2. Synthesis of Dacomitinib Butanoic Acid
While the discovery routes to dacomitinib carried quinazoline inter- mediates through multiple stages, this approach was challenging on the process scale due to the low solubility of quinazolines in organic solvents. The formation of the quinazoline in the final step ameliorated this problem. Toward this end, intermediate 4 underwent a Dimroth rearrangement in the presence of aniline 5 to provide dacomitinib (XXIV). As both 5 and dacomitinib are each unstable under the high temperatures typically employed for Dimroth rearrangements (e.g., refluxing HOAc), these conditions were optimized to proceed at lower temperatures. This reaction represents one of the few reported examples of a Dimroth reaction occurring under such mild conditions. The reaction ultimately occurred at 30 °C in neat acetic acid using 2 equiv of aniline 5 to increase the reaction rate. The reaction was quenched with 2-MeTHF and NaOH, neutralized with triethylamine, and a seeded crystallization isolated the dacomitinib hydrate.
References
[1] Jinyao Zhang. “Efficacy of dacomitinib in patients with EGFR-mutated NSCLC and brain metastases.” Thoracic Cancer 12 24 (2021): 3407–3415.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
You may like
Related articles And Qustion
See also
Lastest Price from Dacomitinib (PF299804) manufacturers

US $0.00/g2025-04-21
- CAS:
- 1110813-31-4
- Min. Order:
- 100g
- Purity:
- 99%min
- Supply Ability:
- 1000g
US $0.00-0.00/g2025-04-20
- CAS:
- 1110813-31-4
- Min. Order:
- 10g
- Purity:
- 99% HPLC
- Supply Ability:
- 1000kg