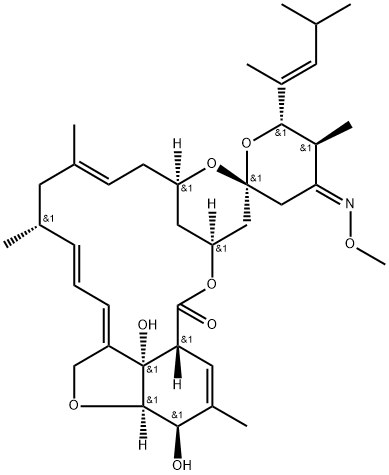
A macrocyclic lactone anthelmintic: Moxidectin
Overview
Moxidectin was developed by the nonprofit firm Medicines Development for Global Health (MDGH). It was approved by the USFDA in 2018 for treating river blindness, also called onchocerciasis, in patients aged 12 years and older. The larvae cause river blindness (microfilariae) of a parasitic worm, Onchocerca volvulus, which manifests as severe itching, disfiguring skin conditions, and visual impairment, including permanent blindness. Nearly 200 million people are at risk of river blindness, with 99% of patients living in sub-Saharan Africa.
As a macrocyclic lactone anthelmintic similar in structure to the avermectins, it is frequently used off-label for gastrointestinal parasite prevention and treatment in South American camelids. Haemonchus contortus (H. contortus), a hemophagic gastrointestinal nematode of the third stomach compartment, causes debilitating anemia and hypoproteinemia in South American camelids and small ruminants. This parasite can rapidly infect the environment and become a herd problem due to its high egg-laying capacity[1].
Mechanism of action
Although the actual mechanism of action is unknown, studies with other nematodes suggest that moxidectin binds to a parasite's glutamate-gated ion channels (GluCl), γ-amino-butyric acid (GABA) receptors, and/or ATP-binding cassette (ABC) transporters. This induces increased permeability, leading to an influx of chloride ions and flaccid paralysis of the parasite. Moxidectin is active against micro-filariae of Onchocerca volvulus but does not kill the adult worms. The drug has a longer half-life than ivermectin, the current standard of care, and offers an alternative for managing antiparasitic drug resistance.
Synthetic method
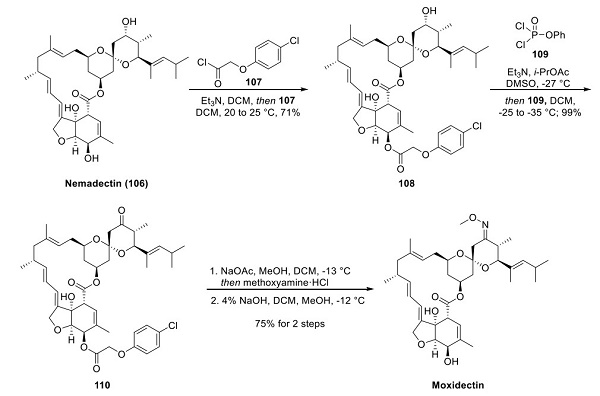
Moxidectin is a 16-membered macrocyclic lactone of the milbemycin class, presenting significant structural synthetic challenges. However, the starting material for synthesizing the drug is highly functionalized macrolactone nemadectin, a fermentation product of Streptomyces cyanogriseus ssp. noncyanogenus. Selective protection of the nemadectin C-25 allylic hydroxyl group with 4-chlorophenoxy acetyl chloride 107 furnished 108. The choice of this protecting group was driven by improved stability and more straightforward purification (recrystallization) of the intermediates. The remaining C-10 secondary alcohol in 108 was oxidized using modified Pfitzner−Moffat conditions using phenyl phosphor- odichloridate (109) to yield ketone 110. Oxime formation and selective saponification of the ester-protecting group furnished moxidectin. It should be noted that the oxime retains the (E)-configuration throughout the final two steps of the molecule's synthesis [2].
References
[1] Christine M. Cocquyt . “Pharmacokinetics of moxidectin in alpacas following administration of an oral or subcutaneous formulation.” Research in veterinary science 105 (2016): Pages 160-164.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
You may like
See also
Lastest Price from Moxidectin manufacturers
US $0.00-0.00/kg2025-12-09
- CAS:
- 113507-06-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg
US $0.00/kg2025-11-21
- CAS:
- 113507-06-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise