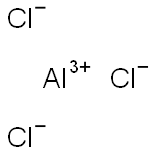
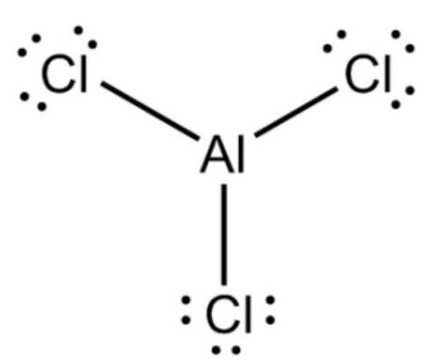
Is AlCl3 ionic or covalent?
Aluminum chloride (AlCl3) is a compound that exhibits characteristics of both ionic and covalent bonding, making it somewhat ambiguous in terms of its classification.
AlCl3 is a compound formed between a metal and non-metal. Al3+ is very polarising due to its high charge density. It is able to pull the electron cloud of chloride to such a great extent that there is orbital overlap between Al and Cl.
The bond between Al and Cl is covalent and hence AlCl3 is a simple molecule. According to Fajan's rule with increasing size of the anion and with decreasing size of the cation, ionic character of the compound decreases while the covalent character increases.
Here since the cation is same in both the compounds, the size of the anion decides the nature of the compound.
So AlCl3 is covalent.
You may like
Related articles And Qustion
See also
Lastest Price from Aluminum chloride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 7446-70-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $2.30/Kg/Bag2025-04-11
- CAS:
- 7446-70-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20tons