Why is AlCl3 a Nonpolar molecule?
Description
Aluminum chloride (AlCl3) is a yellowish or grayish-white crystalline powder with a sharp odor. It is used as a chemical intermediate for Aluminum compounds, as a catalyst for cracking petroleum, preserving wood, and in medications, disinfectants, cosmetics, photography, and textiles. It is noteworthy as a Lewis acid and is extensively used in the Friedel–Crafts alkylation and acylation of aromatic hydrocarbons.
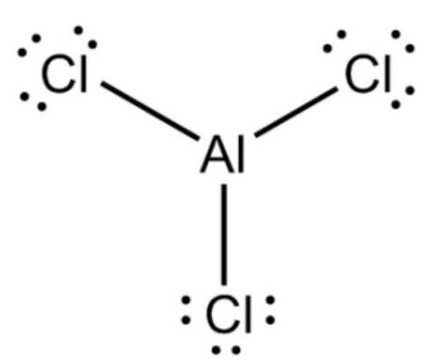
The Lewis dot structure
The Lewis dot structure of AlCl3 is shown below. In the AlCl3 Lewis structure, one atom of aluminum (Al) and three atoms of chlorine (Cl). The number of valence electrons in Al is 3; in Cl, it is 7. The total number of valence electrons in the AlCl3 Lewis structure is 24. The aluminum central atom shares 6 valence electrons through the three single bonds attached to it. It does not contain lone pairs of electrons.
The molecular geometry
The molecular geometry of AlCl3 is trigonal planar because the central atom of aluminum is bonded with three chlorine atoms, and it contains no lone pair; this means distortion around the central position will not happen because of no lone pair. Hence, the three bonded atoms(chlorine) are arranged like a triangle around the central atom(aluminum).
Nonpolar molecule
For Al-Cl bond:
The electronegativity difference (ΔEN) = 3.16 – 1.61 = 1.55
This value lies between 0.4 and 1.7, which indicates that the bond between Aluminum (Al) and Chlorine (Cl) is polar.
Hence, each Al-Cl bond is a polar covalent bond.
However, AlCl3 is a nonpolar molecule because each Al-Cl bond is directed at an angle of 120° to each other in a plane; hence, canceling the dipole moment generated along these bonds is easy. Therefore, no dipole moment is generated in the AlCl3 molecule. In addition, the molecular geometry of AlCl3 is very symmetrical since no lone pair is present on the central atom that can cause distortion in a molecule, so the charges are distributed uniformly all over the atoms. Hence, the dipole generated in the AlCl3 molecule will easily cancel out each other, leaving this molecule nonpolar.
You may like
Related articles And Qustion
See also
Lastest Price from Aluminum chloride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 7446-70-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $1.10/g2025-04-17
- CAS:
- 7446-70-0
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min