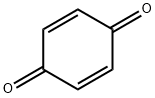
Is 1,4-benzoquinone a toxicity compound?
Yes, 1,4-Benzoquinone is a toxic metabolite found in human blood and can be used to track exposure to benzene or mixtures containing benzene and benzene compounds, such as petrol.
Description
1,4-Benzoquinone (or, less formally, “quinone”) is a yellow crystalline solid with a chlorine-like odor. It is the simplest member of the quinone family of conjugated dienediones with several uses in organic chemistry. It undergoes typical ketone and olefin reactions, but its predominant use is an oxidizing agent.
Chemical property
1,4-benzoquinone is the simplest member of the class of 1,4-benzoquinones, obtained by the formal oxidation of hydroquinone to the corresponding diketone. It has a role as a cofactor, a human xenobiotic metabolite, and a mouse metabolite. 1,4-benzoquinone is expected to be non-polar in the ground state. However, the literature shows a small dipole moment of 0.67 for the compound. As ordinarily formulated, P-Benzoquinone is sufficiently symmetrical to be non-polar, yet it appears to be slightly, but definitely, polar.
Toxicity
Benzene is a colorless, volatile liquid hydrocarbon found in coal tar and petroleum and is used to make numerous chemical products, including detergents, insecticides, and motor fuels. However, it is a common environmental toxin. The primary benzene metabolite to cause genomic damage is 1,4-benzoquinone (BQ), which causes hematopoietic cancers like myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML)[1].
BQ-induced damage efficiently stalls replication forks yet poorly induces ATR/DNA-PKCS responses. Furthermore, the BQ-induced γH2AX and 53BP1foci pattern is consistent with the formation of poly(ADP-ribose) polymerase 1 (PARP1)-stabilized regressed replication forks. At a biochemical level, BQ inhibited topoisomerase 1 (topo1)-mediated DNA ligation and nicking in vitro, thus providing a mechanism for the cellular phenotype.
1,4-benzoquinone is genotoxic and mutagenic[2]. Bone marrow stem cells are targets for benzene-induced cytotoxicity and DNA damage that could result in changes to the genome of these progenitor cells, thereby leading to hematopoietic disorders and cancers. CD34(+) HPC from 10 men and 10 women were exposed to 0, 1, 5, 10, 15, or 20 microM of 1,4-BQ and analyzed 72 h later. Apoptosis and cytotoxicity were dose-dependent, with exposure to 10 microM 1,4-BQ resulting in approximately 60% cytotoxicity relative to untreated controls. A significant increase in micronucleated CD34(+) cell percentage was detected in cultures treated with 1,4-BQ. In addition, the p21 mRNA level was elevated in 1,4-BQ-treated cells, suggesting that human CD34(+) cells utilize the p53 pathway in response to 1,4-BQ-induced DNA damage. However, there were no significant changes in mRNA levels of the DNA repair genes ku80, rad51, xpa, xpc, and ape1, as well as p53, following treatment with 1,4-BQ. Although interindividual variations were evident in the cellular response to 1,4-BQ, there was no gender difference in the response overall. These results show that human CD34(+) cells are sensitive targets for 1,4-BQ toxicity that use the p53 DNA damage response pathway in response to genotoxic stress.
References
[1] Mi Young Son. “A mechanism for 1,4-Benzoquinone-induced genotoxicity.” Oncotarget 7 29 (2016): 46433–46447.
[2] Diane J Abernethy. “Human CD34+ hematopoietic progenitor cells are sensitive targets for toxicity induced by 1,4-benzoquinone.” Toxicological Sciences 79 1 (2004): 82–9.
You may like
Related articles And Qustion
See also
Lastest Price from 1,4-Benzoquinone manufacturers

US $1.00/KG2025-04-21
- CAS:
- 106-51-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 106-51-4
- Min. Order:
- 1kg
- Purity:
- 99.00%
- Supply Ability:
- 100tons