Reactions and applications of 1,4-Benzoquinone
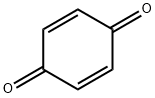
1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde.1,4-benzoquinone is the simplest member of the class of 1,4-benzoquinones, obtained by the formal oxidation of hydroquinone to the corresponding diketone. It is a metabolite of benzene. It has a role as a cofactor, a human xenobiotic metabolite and a mouse metabolite.

Reactions and applications
Quinone is mainly used as a precursor to hydroquinone, which is used in photography and rubber manufacture as a reducing agent and antioxidant.Benzoquinonium is a Skeletal muscle relaxant, ganglion blocking agent that is made from benzoquinone.
Organic synthesis
It is used as a hydrogen acceptor and oxidant in organic synthesis. 1,4-Benzoquinone serves as a dehydrogenation reagent. It is also used as a dienophile in Diels Alder reactions.
Benzoquinone reacts with acetic anhydride and sulfuric acid to give the triacetate of hydroxyquinol.This reaction is called the Thiele reaction or Thiele–Winter reactionafter Johannes Thiele, who first described it in 1898, and after Ernst Winter, who further described its reaction mechanism in 1900.
An application is found in this step of the total synthesis of Metachromin A:
An application of the Thiele reaction, involving a benzoquinone derivative.
Benzoquinone is also used to suppress double-bond migration during olefin metathesis reactions.
An acidic potassium iodide solution reduces a solution of benzoquinone to hydroquinone, which can be reoxidized back to the quinone with a solution of silver nitrate.
Due to its ability to function as an oxidizer, 1,4-benzoquinone can be found in methods using the Wacker-Tsuji oxidation, wherein a palladium salt catalyzes the conversion of an alkene to a ketone. This reaction is typically carried out using pressurized oxygen as the oxidizer, but benzoquinone can sometimes preferred. It is also used as a reagent in some variants on Wacker oxidations.
1,4-Benzoquinone is used in the synthesis of Bromadol and related analogs.
Metabolism
1,4-Benzoquinone is a toxic metabolite found in human blood and can be used to track exposure to benzene or mixtures containing benzene and benzene compounds, such as petrol. The compound can interfere with cellular respiration, and kidney damage has been found in animals receiving severe exposure. It is excreted in its original form and also as variations of its own metabolite, hydroquinone.
Benzoquinone compounds are a metabolite of paracetamol.
Safety
1,4-Benzoquinone is able to stain skin dark brown, cause erythema (redness, rashes on skin) and lead on to localized tissue necrosis. It is particularly irritating to the eyes and respiratory system. Its ability to sublime at commonly encountered temperatures allows for a greater airborne exposure risk than might be expected for a room-temperature solid.
You may like
Related articles And Qustion
See also
Lastest Price from 1,4-Benzoquinone manufacturers

US $1.00/KG2025-04-21
- CAS:
- 106-51-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 106-51-4
- Min. Order:
- 1kg
- Purity:
- 99.00%
- Supply Ability:
- 100tons