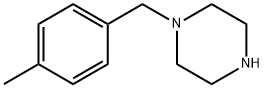
Biological properties of 1-(4-Methylbenzyl) piperazine
The piperazines are a broad class of chemical compounds with important pharmacological properties [1]. Piperazine is an organic compound that consists of a six-member cyclic secondary diamine with the nitrogen in the 1, 4 – position, which makes piperazine a weak base. The nitrogen in piperazine ring plays an important role in biological research and drug manufacturing industry, including the preparation of anthelmintic, antiallergenic, antibacterial, antihistamic, antiemetic and antimigraine agents.
1-(4-Methylbenzyl) piperazine (MBPZ), the substituents attached to benzene ring are at para position with respect to each other, has excellent biological properties in anti-helminths, antihistaminic, anticancer, antidepressant and anti-microbial activity because of the chemical structure [2]. The biological property of 1-(4-Methylbenzyl) piperazine could be predicted with BAITOC (Bioactivity Information to Organic Chemist) software. In 2017, researches utilized the software and predicted MBPZ could inhibit Bacillus cereus based on the special structure of MBPZ.
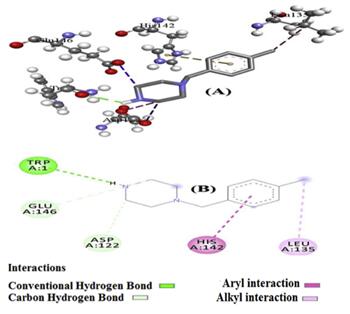
In the prediction, as shown in the above graph, TRP1 of the protein forms conventional hydrogen bonds with-NH group, and carbon hydrogen bonding is observed by the interaction of carbon atoms of the piperazine ring with GLU 146 and ASP122. Meanwhile, the aromatic ring of MBPZ was observed the interaction with HIS 142 of the protein, and alkyl interaction takes place between LEU 135 of the protein and -CH3 group of MBPZ. In charge analysis, it was observed that C2, C3, C5 and C7 of aromatic ring were electronegative and act as electrophilic sites, which contributed the interaction with the protein. The results predicted that the compound might exhibit inhibitory activity against Bacillus cereus and may act as an anti-bacterial agent [2].
In biological application, 1-(4-Methylbenzyl) piperazine also shows good stability. Timothy Lau studied the stability of synthetic piperazines in human blood under various storage conditions over time [3]. All samples were prepared by spiking certified reference standards of eight synthetic piperazines into certified drug-free human blood independently with same concentration. These analytes included: 1-benzylpiperazine (BZP), 1-(4-fluorobenzyl)-piperazine(FBZP), 1-(4-methylbenzyl)-piperazine(MBZP), 1-(4-methoxyphenyl)-piperazine(MeOPP), 1-(para-fluorophenyl)-piperazine(pFPP), 1-(3-chlorophenyl)-piperazine(mCPP), 2,3-dichlorophenylpiperazine(DCPP), and 1-(3-trifluoromethylphenyl)-piperazine (TFMPP). All tested samples were stored at room temperature (~20°C), 4°C and -20°C for 1, 3, 6, 9 and 12 months respectively in dark sealed containers. Results revealed that BZP, MBZP and FBZP were more stable than phenyl piperazines over time under all storage conditions, in which MBZP (1-(4-Methylbenzyl) piperazine) was consistently more stable and still had more than 70% remaining after 12 months.
Although MBZP (1-(4-Methylbenzyl) piperazine) has biological properties, it generally acts as synthetic intermediate, such as the synthesis of 3-Desmethyl 4-Methyl Meclizine Dihydrochloride, a metabolite of Meclizine (2HCl monohydrate) which is an antiemetic. 1-(4-Methylbenzyl) piperazine is also used in the synthesis of inhibitors of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE).
MBZP (1-(4-Methylbenzyl) piperazine) also can be introduced to existing chemical to get a new one with high biological performance. Phenazine-1-carboxylic acid (PCA) as a natural product which has significant inhibition effects against many soil-borne fungal phytopathogens in agricultural application. In order to find new higher fungicidal activities lead compounds, and develop new eco-friendly agrochemicals, piperazines structure was introduced into PCA structure and get a series of piperazine derives, which exhibited certain in vitro fungicidal activities. Particularly, new modified compounds exhibited the activity against all the tested pathogenic fungi, which showed more potent activities than PCA. This result provided a highly active lead compound for the further structure optimization design and extended the application of piperazine structure chemicals also [4].
Reference
[1] https://en.wikipedia.org/wiki/Piperazine
[2] K. Subashini, S. Periandy. Spectroscopic (FT-IR, FT-Raman, UV, NMR, NLO) investigation and molecular docking study of 1-(4-Methylbenzyl) piperazine, Journal of Molecular Structure, Volume 1134, 15 April 2017, Pages 157-170
[3] Timothy Lau; Raquel LeBlanc; Sabra Botch-Jones, Stability of Synthetic Piperazines in Human Whole Blood. Journal of analytical toxicology, 2018-03-01
[4] Han, Fei; Yan, Ru; Zhang, Min; Xiang, Zhu; Wu, Qinglai; Li, Junkai, Synthesis and bioactivities of phenazine-1-carboxylic piperazine derivatives, Natural Product Research (2019), Ahead of Print