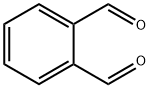
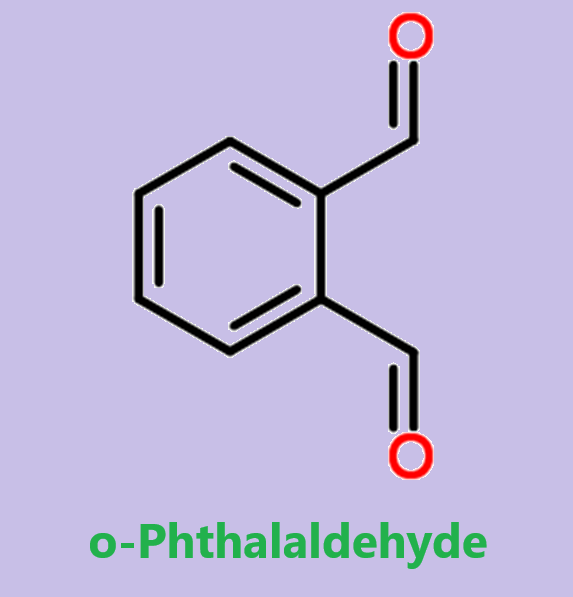
Introduction of Ortho-phthalaldehyde
Abstract
In the existence of appropriate amount of disodium ethylenediaminetetraacetate (EDTA), precipitation would not occur in seawater and other natural waters even if the sample solution was adjusted to strong basicity, and the NH3-OPA-sulfite reaction at the optimal pH range could be used to determine ammonium in natural waters. Based on this, a modified o-phthalaldehyde fluorometric analytical method has been established to determine ultratrace ammonium in natural waters. Experimental parameters, including reagent concentration, pH, reaction time, and effect of EDTA, were optimized throughout the experiments based on univariate experimental design. The results showed that the optimal pH range was between 10.80 and 11.70. EDTA did not obviously affect the fluorometric intensity. The linearity range of the proposed method was 0.032–0.500?μmol/L, 0.250–3.00?μmol/L, and 1.00–20.0?μmol/L at the excitation/emission slit of 3?nm/5?nm, 3?nm/3?nm, and 1.5?nm/1.5?nm, respectively. The method detection limit was 0.0099?μmol/L. Compared to the classical OPA method, the proposed method had the advantage of being more sensitive and could quantify ultratrace ammonium without enrichment.

1. Introduction
Ammonia nitrogen consists of ammonia (NH3) and ammonium (NH4) in natural waters. Ammonium is predominant when the pH is below 8.75, and ammonia is predominant when pH is above 9.75 [1]. Ammonium is the main species in the pH range of most natural waters and is an essential nutrient in aquatic ecosystems [2]. The concentration of ammonium is usually more than micromolar level in mostly continental water and coastal seawater, even up to millimolar level due to environmental pollution [3]. However, it is less than micromolar level, even down to nanomolar level in ocean water [4]. The accurate measurement of ammonium concentrations is fundamental to understanding nitrogen biogeochemistry in aquatic ecosystems. The most common techniques used to measure ammonium in freshwater and seawater are the indophenol blue method [5, 6] and o-phthalaldehyde (OPA) fluorometric method [7–15]. The o-phthalaldehyde (OPA) fluorometric method is much more sensitive than the indophenol blue method. It has attracted a great deal of attention of scientists. In 1971, it was firstly reported that OPA could react with amino acid and ammonium in the existence of mercaptoethanol to produce a strongly fluorescent compound [7]. In 1989, the reaction was modified by replacing mercaptoethanol with sulfite, organic amine compounds did not interfere in the determination, and an OPA fluorometric method with higher sensitivity and selectivity was developed for ammonium by Genfa and Dasgupta [8]. Afterwards, the method was further modified for the determination of ammonium in seawater [9–11] and was developed for shipboard using flow injection technology [12–14]. Recently, the sensitivity of the OPA method was further remarkably improved to determine ocean surface water by combining fluorescence detection with flow analysis and solid phase extraction [15]. The main analytical parameters of the OPA methods mentioned above were listed in Table 1. The lower limit of quantitation (LOQ) listed in the table was the lowest concentration of the working range in the corresponding references. Table 1 showed that the sensitivity of the reported methods was gradually improved and the LOQ was decreased using advanced technology such as flow injection, autonomous batch, and solid extraction technology.
The fluorometric reaction of NH3-OPA-sulfite was found to be pH dependent by much reported work. The optimal pH was reported at 11 by Amornthammarong and Zhang in 2008 [12]. However, when pH was more than 10.4, precipitation easily occurred due to the existence of metal ions in natural water sample. To avoid precipitation of the metal ions, much work had to control the pH about 9.3 using sodium tetraborate solution as buffer [9, 10, 14, 15]. According to the reported data, the sensitivity of the method at pH = 9.3 was several times less than that at pH = 11. Therefore, it was considered that the method sensitivity could be also proved by changing the reaction pH. In this work, a modified NH3-OPA-sulfite reaction using EDTA-NaOH as buffer was described. In the existence of appropriate amount of EDTA, precipitation would not occur in natural water even if the solution was adjusted to strong basicity, and the NH3-OPA-sulfite reaction under the optimal pH condition could be used to determine ammonium in natural waters. Based on this, a new modified o-phthalaldehyde fluorometric analytical method was established. The method is highly sensitive for determination of ammonium in natural waters without enrichment.
2. Experimental
2.1. Reagents and Solutions
All the chemicals used in this study were of analytical grade, supplied by Aladdin Chemical Reagent Co., China, unless stated otherwise. All solutions were prepared in ultrapure water (resistivity 18.2 MΩcm).
Standard Solution. Ammonium standard stock solution (1000?mg?N/L) was purchased from Aladdin Chemical Reagent Co. Ammonium standard substock solution (10?mmol /L) was prepared monthly by diluting the stock solution with ultrapure water. The stock and substock solutions were stored at 4°C in a refrigerator while not in use. Ammonium working solution (0.1?mmol /L) was prepared daily by diluting 1.0?mL of the substock solution to 100?mL with ultrapure water.
Fluorescent Reagent Solution (R1). 10.6?g/L OPA solution (Reagent A) was made by dissolving 2.65?g of OPA in 50?mL of methanol (HPLC grade) and diluted to 250?mL with ultrapure water. 2.5?g/L sodium sulfite solution (Reagent B) was made by dissolving 1.25?g of Na2SO3 in 500?mL ultrapure water and adding 0.20?mL HCHO to prevent the solution from being oxidized. To reduce reagent blank, fluorescent reagent solution (R1) was prepared daily by mixing equal volumes of Reagent A with Reagent B and allowed to stand in room temperature for at least 2 hours and then passed through the OASIS HLB 6?cc/200?mg cartridge (Waters Corp., Millford, MA) at a flow rate of 3.0?mL/min before use.
EDTA-NaOH Buffer Solution (R2) and NaOH Solution. EDTA-NaOH buffer solution (R2) was made by dissolving 46.6?g disodium ethylenediaminetetraacetate (EDTA, ACS grade) and 8.75?g NaOH (ACS grade) in 500?mL ultrapure water. NaOH solution (R3) was made by dissolving 10.0?g NaOH (ACS grade) in 500?mL ultrapure water. Sodium tetraborate buffer solution (R4) was prepared by dissolving 3.75?g Na2B4O710H2O in 500?mL ultrapure water. These above-mentioned three solutions were separately boiled for several ten minutes to remove ammonia till the final volume of the solution was reduced to half of the original volume and were immediately cooled in water bath and tightly sealed in a polypropylene bottle. The bottles were double-bagged using polyethylene bag while not in use.
All vessels used in the experiments were firstly soaked with 1?molL?1??HCl for more than 12 hours and cleaned with RO water and then were soaked with 1?molL?1??NaOH at least for 12 hours and cleaned thoroughly with ultrapure water before use.
2.2. Analytical Procedures
2.2.1. The Proposed Method
20?mL of standard ammonium solution or sample solution with a concentration range of 0.032–15.0?μmol/L was exactly measured into a polypropylene bottle. Appropriate amounts of fluorescent reagent (R1) and buffer (R2) were added into the solution. The concentrations of OPA, sodium sulfite, and EDTA in the final solution were 0.691?g/L, 0.163?g/L, and 5.77?mmol/L, respectively. The pH of the final solution was controlled in the range of 11.0–11.4 by adding appropriate amounts of NaOH solution (R3). After all the reagents were added, the mixed solution was tightly sealed and allowed to react for 50 minutes at room temperature. At least ten samples could be determined at the same time. The fluorescence intensity (FI) was measured on a fluorescence spectrophotometer (RF-5301PC, SHIMADZU Co., Ltd., Japan) with excitation wavelength set at 361?nm and emission wavelength at 423?nm.
2.2.2. The Classical OPA Method
Based on the method of [10, 15], 20?mL of standard ammonium solution or sample solution with a concentration range of 0.250–2.00?μmol/L was exactly measured into a polypropylene bottle. Both 3?mL of fluorescent reagent (R1) and 5?mL of sodium tetraborate buffer (R4) were added to the solution. After all the reagents were added, the mixed solution was tightly sealed and allowed to react for 60 minutes at room temperature. The pH of the reaction solution is about 9.3. The fluorescence intensity (FI) was measured in the same instrument as the proposed method.
3. Results and Discussion
3.1. Parameters Optimization
The OPA-NH3-sulfite reaction may be affected by the parameters OPA and sodium sulfite concentrations, reaction time, and pH. These parameters had been optimized by much work [7–10]. However, the reaction system in this work was different from previous works due to adding EDTA-NaOH buffer solution, so they were optimized based on a univariate experimental design again.
3.1.1. Spectral Characteristics of the Reaction Production
0.25?μmol/L standard ammonium solution was allowed to react with OPA and sodium sulfite in the presence of EDTA-NaOH buffer according to Section 2.2.1. The excitation and emission spectra of the reaction product are showed in Figure 1.
Related articles And Qustion
See also
Lastest Price from o-Phthalaldehyde manufacturers

US $8.00/KG2025-06-10
- CAS:
- 643-79-8
- Min. Order:
- 10KG
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $10.00/kg2025-04-21
- CAS:
- 643-79-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt