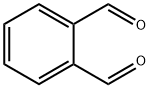
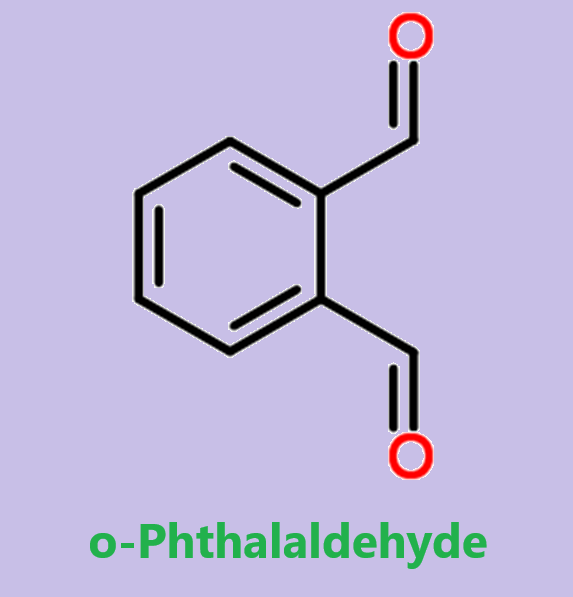
Phthalaldehyde: A Versatile Compound in Modern Chemistry and Biotechnology
Phthalaldehyde (OPA) is a pale yellow solid, it is a building block in the synthesis of heterocyclic compounds and a reagent in the analysis of amino acids. OPA dissolves in water solution at pH < 11.5. Its solutions degrade upon UV illumination and exposure to air.

Properties
Phthalaldehyde (OPA) is a non-fluorescent compound that forms a fluorescent isoindole derivative in the presence of both a primary amine and a free thiol moiety.
Biochemistry uses
Phthalaldehyde (OPA) is a high-level disinfectant that received FDA clearance in October 1999. It contains at least 0.55% 1,2-benzenedicarboxaldehyde or OPA, and it has supplanted glutaraldehyde as the most commonly used "aldehyde" for high-level disinfection in the United States. OPA solution is a clear, pale-blue liquid with a pH of 7.5.
Phthalaldehyde (OPA) is the most common derivatizing reagent in HPLC-PCD-FL. Due to its rapid reactions, high quantum fluorescence of the derivatives and its low cost, OPA is usually used for the tagging of primary amines or thiols under flow conditions. Phthalaldehyde reacts with a primary NH2-group in the presence of a nucleophilic compound yielding a fluorescent iso-indole derivative. There are also compounds that can react with OPA through alternative mechanisms, i.e. without the need of nucleophilic compound, such as histidine, histamine and glutathione at a mildly basic medium and hydrazine in acidic medium. Some applications of post-column derivatization using OPA are the following: determination of amino acids and polyamines, active pharmaceutical ingredients, antioxidants (glutathione) and vitamin B5 (pantothenic acid) in biological fluids.[1]
[1] Apostolia Tsiasioti, Paraskevas D. Tzanavaras. “Developments in on-line, post separation sample manipulation in the last 22 years: Pharmaceutical and biomedical applications.”Journal of pharmaceutical and biomedical analysis 235 (2023): Article 115654.
References:
[1] APOSTOLIA TSIASIOTI P D T. Developments in on-line, post separation sample manipulation in the last 22 years: Pharmaceutical and biomedical applications[J]. Journal of pharmaceutical and biomedical analysis, 2023, 235. DOI:10.1016/j.jpba.2023.115654.You may like
Related articles And Qustion
See also
Lastest Price from o-Phthalaldehyde manufacturers

US $8.00/KG2025-06-10
- CAS:
- 643-79-8
- Min. Order:
- 10KG
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $10.00/kg2025-04-21
- CAS:
- 643-79-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt