o-Phthalaldehyde: derivatives and applications
General Description
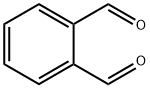
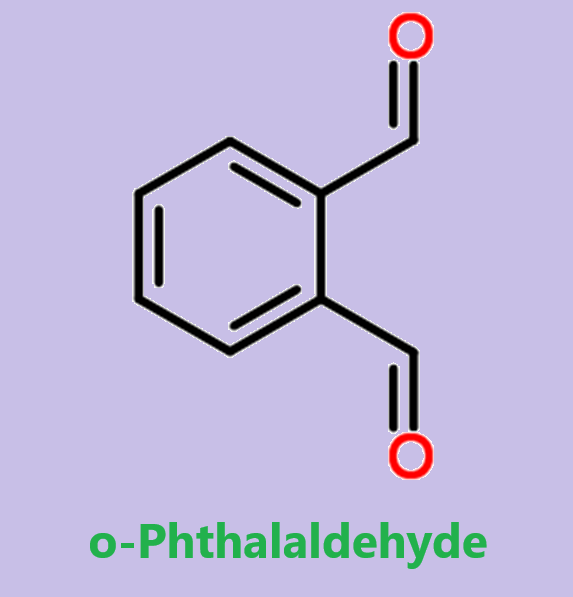
o-Phthalaldehyde is a colorless or white crystalline powder with a strong characteristic odor. It is used as a reagent for derivatives of amino acids, amines, and related compounds. o-Phthalaldehyde and its derivatives have various characteristics and applications. o-Phthalaldehyde derivatives, such as 4-chloro and 4-bromo phthalaldehyde, can be synthesized through alternative approaches due to problematic direct halogenation. These derivatives exhibit improved thermal stability compared to o-phthalaldehyde itself. They can be polymerized via cationic polymerization using BF3·OEt2 at low temperatures. o-Phthalaldehyde derivatives are used in photoresists for lithography, where they become highly sensitive when combined with a photoacid generator (PAG). Under radiation, the PAG releases acids that rapidly depolymerize the o-phthalaldehyde chains, allowing for high-resolution image formation. o-Phthalaldehyde can also undergo complete depolymerization through acid-catalyzed hydrolysis, making it useful in self-development photoresists. Additionally, o-phthalaldehyde shows potential as a mechanically responsive material, undergoing depolymerization triggered by mechanical force. This characteristic opens up possibilities for recyclable materials and mechanically triggered delivery systems.

Figure 1. o-Phthalaldehyde
Derivatives
o-Phthalaldehyde derivatives have several characteristics. Electron withdrawing groups stabilize acetals, making them suitable for testing in polymerization processes. However, direct halogenation of o-phthalaldehyde is problematic, so an alternative approach was proposed to synthesize 4-chloro and 4-bromo phthalaldehyde derivatives. These derivatives can be polymerized through cationic polymerization using BF3·OEt2 at -78°C, but not through anionic polymerization with nBuLi. The thermal stability of these derivatives, such as poly(4-chlorophthalaldehyde) (PCPA), poly(4-bromophthalaldehyde) (PBPA), and poly(4-trimethylsilylphthalaldehyde) (PSPA), is improved compared to unsubstituted o-phthalaldehyde. TGA studies showed that these polymers are stable up to 200°C. Interestingly, PSPA prepared by cationic polymerization exhibited lower thermal stability than PSPA prepared using anionic polymerization. DSC studies revealed that o-phthalaldehyde and its derivatives do not exhibit a glass transition below the decomposition temperature. With the addition of a photoacid generator (PAG), these derivatives show high thermal stability and potential high-resolution capability in photolithography. Furthermore, the presence of double chlorine substitution in PCl2PA enhances the stability of the polymer, preventing undesired reactions. However, PCl2PA can still depolymerize completely from head to tail upon trigger cleavage at room temperature. 1
Applications
Photoresists
o-Phthalaldehyde is a versatile material used as a base for photoresists in lithography. It is soluble in common solvents and can form homogeneous films. o-Phthalaldehyde itself is not photosensitive, but when combined with a photoacid generator (PAG), it becomes highly sensitive. Under UV, X-ray, or e-beam irradiation, the PAG undergoes photolysis, releasing strong acids that initiate rapid depolymerization of o-phthalaldehyde chains. This chemical amplification process allows for high sensitivity even with low doses of radiation. o-Phthalaldehyde can self-develop into relief images without the need for additional development processes. End-capped o-phthalaldehyde can also function without a PAG, but its thermal decomposition occurs at higher temperatures compared to o-phthalaldehyde/PAG resists which can self-develop at lower temperatures. 2
Self-immolative materials
o-Phthalaldehyde can be depolymerized through acid-catalyzed hydrolysis, but it does not follow the typical end-to-end depolymerization mechanism observed in self-immolative polymers (SIPs). However, o-phthalaldehyde's ability to undergo complete depolymerization has found applications in photoresists. By combining it with a photoacid generator (PAG), o-phthalaldehyde can be rapidly and completely depolymerized upon exposure to UV, X-ray, or e-beam irradiation. This feature enables o-phthalaldehyde-based photoresists to achieve high sensitivity and self-development of relief images without the need for additional development processes. 3
Mechanically responsive materials
o-Phthalaldehyde has shown potential for applications in mechanically responsive materials. It can undergo depolymerization triggered by mechanical force, such as ultrasonic induced cavitation or sonication. The depolymerization occurs along the acetal backbone of o-phthalaldehyde, resulting in the formation of monomers. The use of different solvents can affect the rate of mechanochemical scission, with lower solvent vapor pressure enhancing the efficiency. These findings open up possibilities for o-phthalaldehyde to be used in recyclable or regenerative synthetic materials and for mechanically triggered delivery of materials. 4
Reference
1. Wang F, Diesendruck CE. Polyphthalaldehyde: Synthesis, Derivatives, and Applications. Macromol Rapid Commun, 2018, 39(2).
2. Reichmanis E, Houlihan F, Nalamasu O, Neenan T, Chemical amplification mechanisms for microlithography. Chem Mater, 1991, 3:394.
3. Phillips ST, DiLauro AM. Continuous Head-to-Tail Depolymerization: An Emerging Concept for Imparting Amplified Responses to Stimuli-Responsive Materials. ACS Macro Lett, 2014, 3(4):298-304.
4. Diesendruck CE, Peterson GI, Kulik HJ, Kaitz JA, Mar BD, May PA, White SR, Martínez TJ, Boydston AJ, Moore JS. Mechanically triggered heterolytic unzipping of a low-ceiling-temperature polymer. Nat Chem, 2014, 6(7):623-628.
Related articles And Qustion
See also
Lastest Price from o-Phthalaldehyde manufacturers

US $8.00/KG2025-06-10
- CAS:
- 643-79-8
- Min. Order:
- 10KG
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $10.00/kg2025-04-21
- CAS:
- 643-79-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt