Cabozantinib: mechanism of action, pharmacokinetics and clinical applications
General Description
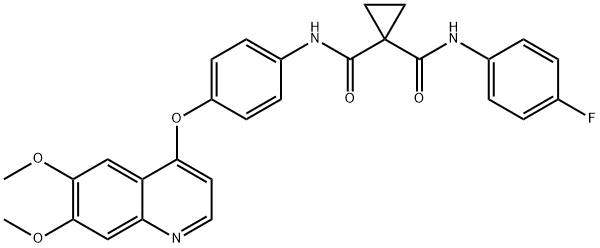
Cabozantinib is a multi-targeted tyrosine kinase inhibitor used to treat thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma. It inhibits VEGF, MET, and TAM kinases, promoting an immune-permissive tumor microenvironment and enhancing antitumor immune responses. The recommended starting dose is 60 mg per day, with adjustments based on individual factors. Steady-state plasma levels are reached in about 15 days, and dose modifications may be necessary due to variability in clearance. Adverse events may require temporary treatment discontinuation. Cabozantinib is primarily metabolized by the liver and can interact with concomitant medications. It has shown efficacy in thyroid cancer, renal cell carcinoma, and gastrointestinal stromal tumors but is associated with adverse effects such as fatigue, diarrhea, and hypertension. Close monitoring and dosage adjustments are important for managing side effects during treatment.
Figure 1. Tablets of cabozantinib
Mechanism of action
Cabozantinib exhibits multiple mechanisms of action in the tumor microenvironment. It targets pathways involved in immunosuppression, such as VEGF, MET, and TAM kinases. By inhibiting VEGF, it reduces the activity of T-regulatory cells and myeloid-derived suppressor cells, while promoting dendritic cell maturation and lymphocyte infiltration. Inhibition of MET and TAM kinases contributes to an immune-permissive microenvironment by decreasing PD-L1 expression on tumor cells and preventing the recruitment of immunosuppressive neutrophils. Additionally, TAM receptors and their ligand GAS6 suppress antigen-presenting cell maturation and reduce the recruitment of cytotoxic T-cells. Activation of AXL on tumor cells by Cabozantinib can further contribute to immune suppression by suppressing MHC class I expression. Preclinical studies suggest that Cabozantinib may enhance the response to immune checkpoint inhibitors through its immunomodulatory effects. Overall, Cabozantinib acts on multiple targets to promote an immune-permissive tumor microenvironment and enhance antitumor immune responses. 1
Pharmacokinetics
Cabozantinib is a medication available in capsule and tablet forms. It is used to treat medullary thyroid cancer, renal cell carcinoma (RCC), and hepatocellular carcinoma (HCC). The recommended starting dose for advanced HCC is 60 mg per day, but it can be adjusted based on individual factors such as concomitant medications and clinical conditions. Steady-state plasma levels of cabozantinib are typically achieved after about 15 days of treatment. Dose modifications may be necessary due to variability in drug clearance among patients. Factors like genetic polymorphisms, hepatic impairment, drug interactions, and food intake can affect clearance and exposure. If adverse events occur, the treatment may be temporarily stopped for up to 7 days. Cabozantinib should be discontinued at least 21 days before surgery. Once the adverse event resolves, the medication can usually be restarted at a reduced dose of 40 mg, and further reduction to 20 mg may be considered. Patients with low clearance are more likely to require dose reduction for tolerability without significantly impacting efficacy. Cabozantinib is primarily metabolized by the liver through the CYP3A4 pathway. Hepatic impairment can moderately increase cabozantinib exposure, but initial dose reduction is not recommended for mild impairment. Limited data is available for severe hepatic impairment or renal impairment. 2
Clinical applications
Cabozantinib, a small-molecule tyrosine kinase inhibitor, has been widely used in the clinical setting due to its efficacy in treating various cancers. It targets multiple kinases, including c-Met, vascular endothelial growth factor receptor (VEGFR), and RET, among others. In particular, cabozantinib has shown promise in the treatment of thyroid cancer, where it has been approved for use in advanced papillary, follicular, and anaplastic thyroid cancers. Additionally, cabozantinib has also shown activity in renal cell carcinoma and gastrointestinal stromal tumors. However, despite its efficacy, cabozantinib is associated with adverse effects such as fatigue, diarrhea, and hypertension. Therefore, it is crucial to monitor patients closely during treatment and adjust the dosage as necessary to manage these side effects. In conclusion, cabozantinib is a valuable therapy for the treatment of various cancers, with particular promise in thyroid cancer. 3
Reference
1. Akalu YT, Rothlin CV, Ghosh S. TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev, 2017, 276:165–77
2. El-Khoueiry AB, Hanna DL, Llovet J, Kelley RK. Cabozantinib: An evolving therapy for hepatocellular carcinoma. Cancer Treat Rev, 2021, 98:102221.
3. Fallahi P, Ferrari SM, Di Bari F, Materazzi G, Benvenga S, Miccoli P, Antonelli A. Cabozantinib in Thyroid Cancer. Recent Pat Anticancer Drug Discov, 2015, 10(3):259-269.
Related articles And Qustion
See also
Lastest Price from Cabozantinib manufacturers

US $0.00/kg2025-07-05
- CAS:
- 849217-68-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $5.00-0.50/KG2025-06-05
- CAS:
- 849217-68-1
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS