Cabozantinib:Efficacy,effectiveness and safety
General Description
Cabozantinib has proven to be an effective treatment for renal cell carcinoma and hepatocellular carcinoma, demonstrating significant improvements in progression-free survival, overall survival, and objective response rate in both randomized clinical trials and real-world studies. Notably, the METEOR and CABOSUN trials underscored its superiority over everolimus and sunitinib in RCC, while the CELESTIAL trial highlighted its efficacy in HCC patients previously treated with sorafenib. Despite a notable incidence of grade 3-4 adverse events such as hypertension, diarrhea, fatigue, and palmar-plantar erythrodysesthesia syndrome, these AEs are manageable with dose adjustments and treatment discontinuations when necessary. This safety and efficacy profile establishes cabozantinib as a valuable option for managing advanced RCC and HCC.

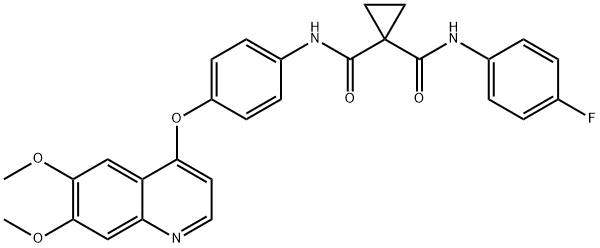
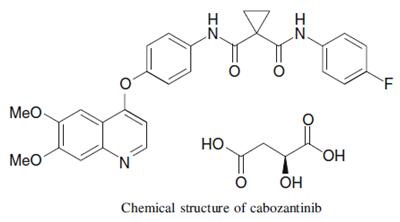
Figure 1. Cabozantinib
Efficacy and effectiveness
Cabozantinib has demonstrated significant efficacy and effectiveness in the treatment of renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC), as evidenced by randomized clinical trials (RCTs) and real-world studies. In RCC, the phase III METEOR trial and phase II CABOSUN trial highlighted cabozantinib's superior efficacy over everolimus and sunitinib, respectively, showing improved progression-free survival (PFS), overall survival (OS), and objective response rate (ORR). The METEOR trial specifically noted a disease control rate (DCR) of 83% for cabozantinib compared to 66% with everolimus, with benefits observed across different patient subgroups, including those with prior nephrectomy, various age groups, and prior immuno-oncology treatments. The CABOSUN trial demonstrated cabozantinib's effectiveness as a first-line therapy, significantly lowering the risk of disease progression compared to sunitinib. For HCC, the phase III CELESTIAL trial showed that cabozantinib significantly increased median OS and PFS over placebo in patients previously treated with sorafenib. This benefit was consistent even in subsets of patients with deteriorating liver function or those who had received prior transarterial chemoembolization (TACE). Real-world evidence supports these findings, indicating similar outcomes in broader patient populations outside of controlled clinical trials. These studies have shown cabozantinib's effectiveness across various lines of therapy in RCC and as a second or later line treatment in HCC, with consistent improvements in OS, PFS, ORR, and DCR. Collectively, the data from both RCTs and real-world studies underscore cabozantinib's role as a valuable therapeutic option for managing advanced RCC and HCC. 1
Safety
The safety profile of cabozantinib, a medication used in the treatment of various cancers including renal cell carcinoma and hepatocellular carcinoma, has been extensively evaluated through randomized clinical trials and real-world studies. In RCC, two RCTs reported that 67-68% of patients experienced grade 3-4 adverse events (AEs) with cabozantinib 60 mg treatment. The most frequent grade 3-4 AEs included hypertension (15-28%), diarrhea (10-11%), fatigue (6-9%), and palmar-plantar erythrodysesthesia syndrome (PPES; 8%). Treatment discontinuation due to AEs occurred in 9-20% of patients, and 46-60% required dose reductions. Real-world studies in RCC showed a lower incidence of grade 3-4 AEs (15-49%) compared to RCTs. Common AEs were similar to those observed in RCTs, including hypertension, diarrhea, fatigue, and PPES. The proportion of patients discontinuing treatment due to AEs ranged from 4% to 16%, and 23% to 69% required dose reductions, aligning with RCT findings. For HCC, the phase III CELESTIAL trial reported grade 3-4 AEs in 68% of patients, with PPES (17%), hypertension (16%), increased AST (12%), fatigue (10%), and diarrhea (10%) being the most common. A real-world study in HCC patients found similar treatment-related grade 3-4 AEs, including fatigue, hand-foot skin reaction, hypertension, and increased ALT. Overall, cabozantinib's safety profile is characterized by manageable AEs, with hypertension, diarrhea, fatigue, and PPES being the most common grade 3-4 AEs across different cancer types. Despite the need for dose adjustments and treatment discontinuations in some cases, these findings suggest that with proper management, cabozantinib remains a viable treatment option for RCC and HCC. 2
Reference
1. Maroto P, Porta C, Capdevila J, et al. Cabozantinib for the treatment of solid tumors: a systematic review. Ther Adv Med Oncol. 2022;14:17588359221107112.
2. McGregor B, Mortazavi A, Cordes L, Salabao C, Vandlik S, Apolo AB. Management of adverse events associated with cabozantinib plus nivolumab in renal cell carcinoma: A review. Cancer Treat Rev. 2022;103:102333.
Related articles And Qustion
See also
Lastest Price from Cabozantinib manufacturers

US $0.00/kg2025-07-05
- CAS:
- 849217-68-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $5.00-0.50/KG2025-06-05
- CAS:
- 849217-68-1
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS