Introduction of Methanesulfonic acid
General description
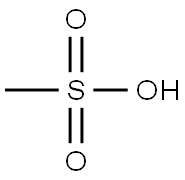
Methanesulfonic acid is an alkanesulfonic acid in which the alkyl group directly linked to the sulfo functionality is methyl. It has a role as an Escherichia coli metabolite. It is an alkanesulfonic acid and a one-carbon compound. It is a conjugate acid of a methanesulfonate. Methanesulfonic acid is an organic compound with chemical formula of ch4o3s. It is soluble in water, alcohol and ether, insoluble in alkanes, benzene and toluene. It does not decompose boiling water and hot alkaline solution, and has strong corrosion effect on metal iron, copper and lead.[1]
Application and pharmacology
Methanesulfonic acid is the raw material of medicine and pesticide. It can also be used as dehydrating agent, coating curing accelerator, fiber treatment agent, solvent, raconization, esterification and polymerization catalyst. Amines are recognized as significant enhancing species on methanesulfonic acid(MSA)-driven new particle formation(NPF).Monoethanolamine (MEA)has been detected in the atmosphere and its concentration could be significantly increased once MEA-based post-combustion CO2 capture technology is widely implemented.Here,weevaluated theenhancingpotentialofMEAon MSA-driven NPF byexamining the formation of MEA-MSA clusters using a combination of quantum.
Synthesis
1.It is obtained by oxidizing methyl thiocyanate with nitric acid: carefully heat nitric acid and effective negative water to 80-88 ℃, add methyl thiocyanate in several times, and the temperature will automatically rise to about 105 ℃. After the reaction is moderated, heat it to 120 ℃ and react for 5 hours to obtain the crude product. Dilute the crude product with exchange water, add 25% barium hydroxide solution, adjust the pH value to 8-9, and filter. The filtrate is deeply reduced to crystallization, and the nitrate is washed away with methanol to obtain barium mesylate. Then add the base into the exchange water and boil it, add sulfuric acid while it is hot, decompose it, filter it, and concentrate the filtrate under reduced pressure until there is no water, so as to obtain the finished product.
2 It is obtained by chlorination, oxidation and hydrolysis of methyl isothiourea sulfate: add methyl isothiourea sulfate into water, inject chlorine at 20-25 ℃, until the color of the solution turns yellow, and there are oil layers at the bottom of the bottle, temperature drops, a large amount of residual chlorine discharged from the exhaust pipe, etc., which is the end point of the reaction. The reaction solution is extracted with chloroform. After drying, chloroform is evaporated under normal pressure at 60-62 ℃, and then distilled under reduced pressure. The fraction at 60-65 ℃ (2.67kpa) is collected to obtain methylsulfonyl chloride. Add 80 ℃ hot water dropwise under stirring and hydrolyze for about 2H until the oil droplets in the reaction solution disappear. The syrup is concentrated with water under reduced pressure, and then the reaction solution is diluted with water under reduced pressure.
Figure 1 the systhesis route of Methanesulfonic acid
Storage and Safety
This product should be sealed in a cool and dry place and stored away from light. This product is packed in 250kg plastic barrel or steel plastic barrel. Store in a cool and ventilated warehouse. Keep away from kindling and heat sources. Isolated from oxidants and alkalis for storage and transportation. Quickly evacuate the personnel in the leakage contaminated area to the safe area, isolate them and strictly restrict their access. Cut off the fire source. It is recommended that emergency treatment personnel wear self-contained positive pressure respirator and acid-base proof work clothes. Cut off the source of leakage as much as possible. If it is liquid, prevent it from flowing into restricted spaces such as sewers and flood drainage ditches. This product has strong irritation to mucous membrane, upper respiratory tract, eyes and skin. Inhalation can cause death due to spasm, inflammation and edema of larynx and bronchus, chemical pneumonia or pulmonary edema. Burning sensation, cough, wheezing, laryngitis, shortness of breath, headache, nausea and vomiting after exposure. Can cause burns.
Reference
1.Shen J., Xie H. & Elm J. et al., "Methanesulfonic Acid-driven New Particle Formation Enhanced by Monoethanolamine: A Computational Study," Environmental Science & Technology, Vol.53, No.24(2019), pp.14387-14397.
You may like
Related articles And Qustion
Lastest Price from Methanesulfonic acid manufacturers

US $1.00/kg2025-04-21
- CAS:
- 75-75-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 75-75-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1000mt