Synthesis of Ethylene carbonate
General description
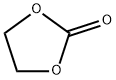
Ethylene carbonate (sometimes abbreviated EC) is the organic compound with the formula (CH 2 O) 2 CO. It is classified as the cyclic carbonate ester of ethylene glycol and carbonic acid. At room temperature (25 °C) ethylene carbonate is a transparent crystalline solid, practically odorless and colorless, and somewhat soluble in water.[1]
Application and pharmacology
Ethylene carbonate (EC) is a typical green chemical product, which is widely used in the fields of gas purification, electrochemistry, organic synthesis, polyester fiber and so on.Physical and chemical properties can be found that vinyl carbonate has relatively high dielectric constant and boiling point, good solubility, tasteless and non-toxic. Therefore, vinyl carbonate can be widely used in textile printing and dyeing, chemical synthesis, petrochemical industry and lithium-ion battery electrolyte.
1.Application of Ethylene carbonate in textile printing and dyeing industry vinyl carbonate has good solubility for polyamide, polyacrylonitrile and other high molecular polymers and resins. Therefore, vinyl carbonate is used as a foaming agent for modifying regenerated cellulose in the industrial field of synthetic fiber; At the same time, vinyl carbonate increases the printing and dyeing properties of synthetic fibers. Because vinyl carbonate can disperse the dye solution more evenly, it is an excellent dispersant in the dyeing process of fiber materials. In the textile industry, the textiles soaked with vinyl carbonate can soften the cloth, making the textiles more comfortable and feel better.
2.Application of Ethylene carbonate in the field of chemical synthesis as an environmental friendly and non-toxic chemical reaction intermediate, vinyl carbonate can replace toxic and polluting compounds to undergo chemical reactions such as alkylation, transesterification and carbonylation, and participate in the synthesis of chemicals.
Synthesis
At present, the synthetic routes of vinyl carbonate mainly include ethylene oxide addition method, phosgene method and ester exchange method, but these traditional synthetic routes are limited due to harsh reaction conditions or serious impact on human body and environment. In recent years, the process of preparing EC by catalytic alcoholysis of urea and ethylene glycol has attracted much attention because of its mild reaction conditions, low cost and easy availability of raw materials and high EC yield. The process is further matched with urea synthesis and vinyl carbonate methanol transesterification reaction to synthesize green solvent dimethyl carbonate (DMC), which can form a complete "zero emission" green closed production chain, which is of great practical significance for realizing the national carbon peak in 2035 and carbon neutralization goal in 2060.
Phosgene synthesis is the main method in the early stage of industrial production of vinyl carbonate. Ethylene glycol, excess pyridine and phosgene are used as the main raw materials, which are generally heated to 70 ℃, and toluene or dichlorotoluene is used as the solvent. The specific reaction equation is as follows:[1]
The Synthesis route 1 of Ethylene glycol
The transesterification method takes ethylene glycol and diethyl carbonate as raw materials and reacts at 120 ℃ ~ 160 ℃ to produce vinyl carbonate. The catalysts used in the general reaction are metal sodium, sodium ethanol and anhydrous potassium carbonate. The reaction formula is as follows:[1]
The Synthesis route 1 of Ethylene glycol
After the transesterification reaction, the product needs to be washed and dried, and the product needs to be distilled under reduced pressure. The loss of vinyl carbonate will be caused in the subsequent treatment, and the operation of the whole process of the reaction is relatively complex. The main reason why this method is eliminated is that the catalyst is more expensive and the yield of the reaction is only about 40%.
Storage and Safety
Galvanized iron bucket or paint baking bucket, with a net weight of 250 ± 0.5kg, can also be packaged by ISO tank or according to the requirements of customers. This product should be stored in a cool, ventilated and dry place and stored and transported according to the provisions of general chemicals.
Reference
1.Wang Longyu: "study on Preparation and reaction performance of catalyst for preparing vinyl carbonate by urea alcoholysis": Beijing Institute of petrochemical technology, 2021.
You may like
Related articles And Qustion
See also
Lastest Price from Ethylene carbonate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 96-49-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 96-49-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000mt/year