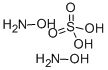Hydroxylamine Sulfate: Application, Toxicity, Preparation
Indication
Hydroxylamine sulfate[1] is an inorganic compound with the chemical formula (NH2OH)2·H2SO4. It is a white crystalline solid that is soluble in water and has a slightly acidic taste. Hydroxylamine sulfate is commonly used in various industrial processes, such as pharmaceuticals, photography, and agriculture. While hydroxylamine sulfate has many applications, it can also be hazardous to handle due to its potential to form explosive materials. Care should be taken when handling this compound and appropriate safety measures should be followed to ensure safe use.

Figure 1 Appearance of hydroxylamine sulfate
Application
Hydroxylamine sulfate is a versatile compound that finds applications in a variety of industries. Its most common use is as a reducing agent in chemical synthesis, where it is used to convert nitro compounds to amines. This reaction is widely employed in the pharmaceutical industry to prepare intermediates for the production of drugs. It is also used in the manufacture of dyes and pigments. It is an important component in the production of sulfur dyes, which are commonly used in the textile industry. Additionally, it is used in the production of photographic films as a developing agent.
The compound's unique properties also make it useful in the water treatment industry. Hydroxylamine sulfate can be added to water to remove dissolved oxygen, which helps prevent corrosion in pipes and other infrastructure. It is also used as a scavenger for hydrogen sulfide, which can cause unpleasant odors and damage to equipment.
In summary, hydroxylamine sulfate is a versatile compound with many applications across multiple industries, including pharmaceuticals, textiles, photography, and water treatment. Its ability to act as a reducing agent, its role in dye and pigment manufacture, and its effectiveness in removing dissolved oxygen and hydrogen sulfide from water make it an important component in many industrial processes.
Toxicity
Hydroxylamine sulfate is a highly toxic compound. It can cause severe skin irritation, burns, and eye damage upon contact. Ingestion or inhalation of hydroxylamine sulfate can lead to respiratory distress, headaches, dizziness, nausea, vomiting, and even death. Chronic exposure to hydroxylamine sulfate may also result in liver and kidney damage. Therefore, it is essential to handle this substance with extreme caution and follow proper safety protocols to avoid any potential harm[2].
Preparation
Synthesis 1: Add 88.5 mmol of cyclohexanone oxime, 11 mmol of deionized water, and 165.5 mmol of sulfuric acid to a glass three-necked bottle equipped with mechanical stirring and cold flaring, respectively, raise the temperature to 60 ℃. After reacting for 1 hour, extract the reaction solution three times with toluene. After the extraction phase is mixed evenly, place the remaining aqueous phase in a low-temperature constant temperature stirring reaction bath at about 5 ℃. Neutralize it with barium hydroxide to a pH of about 6.0-7.0, and then filter under reduced pressure to remove the white barium sulfate precipitate, The filter cake is washed three times with deionized water, and the resulting filtrate is evaporated to obtain a crude hydroxylamine sulfate product. After washing and vacuum drying the crude hydroxylamine sulfate, a white solid product, hydroxylamine sulfate, can be obtained. The conversion rate of cyclohexanone oxime is close to 100%, and the yield of hydroxylamine sulfate solid product is 41.7 %[3].
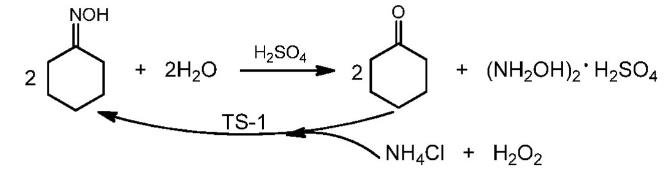
Figure 2 Synthesis of Hydroxylamine sulfate
Synthesis 2:
cyclohexanone oxime and sulfuric acid are the raw materials to prepare hydroxylamine sulfate, hydroxylamine sulfate was prepared by hydrolysis of cyclohexanone oxime under continuous reaction extraction coupling technology. The optimal reaction conditions were obtained under the conditions of using cyclohexane as the extractant, acidoxime ratio of 1:1, rotating speed of 2000-2500 r/min, and equal volume ratio of sulfuric acid solution to cyclohexane. At the same time, it was measured that when the raw material was fed from the heavy phase and extracted through a five-stage series countercurrent reaction, the reaction basically reached equilibrium for 90 minutes. After four cycles of extraction, the conversion rate of cyclohexanone oxime reached 81.90%[4].
References
[1] Zhou Weiping. Hydroxylamine sulfate in urgent need of industrialization [J]. Chemical Abstracts, 2006 (04): 48-50.
[2] Prodanchuk M G, Tsatsakis A M, Prodanchuk G M, et al. Investigation of in vivo toxicity of hydroxylamine sulfate and the efficiency of intoxication treatment by α-tocopherol acetate and methylene blue [J]. Food and Chemical Toxicology, 2013, 61: 227-32.
[3] Xu Yuanyuan, Zhang Dongsheng, Wang Xiaoman, et al. A new reaction process for the synthesis of hydroxylamine sulfate by hydrolysis of cyclohexanone oxime [J]. Chemical Reaction Engineering and Technology, 2017,33 (06): 535-540.
[4] Du Xinyang, Long Bo, Liu Lele, et al. Study on the process of preparing hydroxylamine sulfate with low energy consumption [J]. Journal of Chemical Engineering of Colleges and Universities, 2018,32 (01): 155-160.
You may like
Related articles And Qustion
Lastest Price from Hydroxylamine sulfate manufacturers

US $1.00/KG2025-04-21
- CAS:
- 10039-54-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $3560.00/T2025-03-26
- CAS:
- 10039-54-0
- Min. Order:
- 1T
- Purity:
- 98%
- Supply Ability:
- 1-100mt