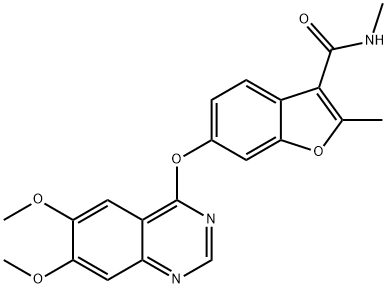
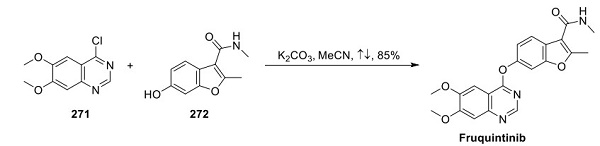
Fruquintinib:Indications,Trials and Side effects
Fruquintinib(HMPL-013) is a small molecular inhibitor of vascular endothelial growth factors (VEGF) -1, -2, and -3, which inhibits tumor growth. It has been approved by the Food and Drug Administration for refractory metastatic colorectal cancer (mCRC).

Indications
Fruquintinib is used for the treatment of adult patients with metastatic colorectal cancer (mCRC) who have been previously treated with or are not considered candidates for available standard therapies, including fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-VEGF agent, an anti-EGFR agent (if RAS wild-type), and either trifluridine-tipiracil or regorafenib.
Trials
Fruquintinib, as an oral tyrosine kinase inhibitor (TKI), effectively suppresses vascular endothelial growth factor/vascular endothelial growth factor receptor (VEGF/VEGFR) signaling and inhibits cell proliferation. Two pivotal randomized controlled trials (RCTs), the FRESCO and the FRESCO2 studies, underscore the potential of fruquintinib as a valuable therapeutic option in later lines of treatment. Real-world studies further substantiate the efficacy and safety of fruquintinib in third line and subsequent treatments. TKIs are frequently used in combination with other drugs in late-line treatment. Fruquintinib, as a novel TKI, lacks large-sample studies supporting its use in combination with other drugs. Preclinical models have indicated potential advantages through the combination of fruquintinib with programmed cell death protein 1 (PD-1) inhibition.Several retrospective studies have demonstrated the potential effectiveness of combining fruquintinib with PD-1 inhibitors. Phase Ib/II studies have delved into the synergy between fruquintinib and sintilimab, revealing promising outcomes. In addition, some studies have started to explore the potential of fruquintinib in combination with chemotherapy. Previous high-quality studies, however, have all been on the use of fruquintinib as a single agent. Reports on the combination of fruquintinib with other drugs have only been from small-sample, single-arm, retrospective cohort studies, and evidence regarding the amalgamation of fruquintinib with chemotherapy is lacking.[1]
Side effects
Hypertension – 38-61% (14-23%)
Fatigue – 25-53% (2.5-12%)
PPE– 19-49% (6-11%)
Diarrhea – 24-25% (3.6-3.7%)
Abdominal Pain – 25-29% (3.7-4%)
Reference
[1] D. Xu .“Fruquintinib in refractory metastatic colorectal cancer: a multicenter real-world study.”ESMO Open 9 11 (2024): Article 103702.
Related articles And Qustion
Lastest Price from Fruquintinib manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 1194506-26-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 20tons

US $0.00-0.00/g2025-04-21
- CAS:
- 1194506-26-7
- Min. Order:
- 2g
- Purity:
- 99%
- Supply Ability:
- 1000kg