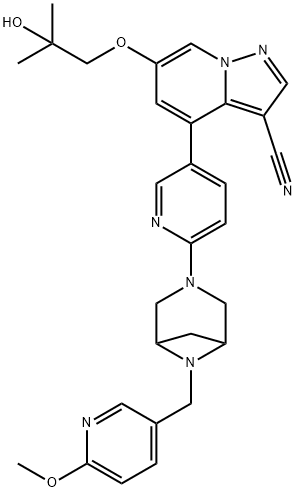
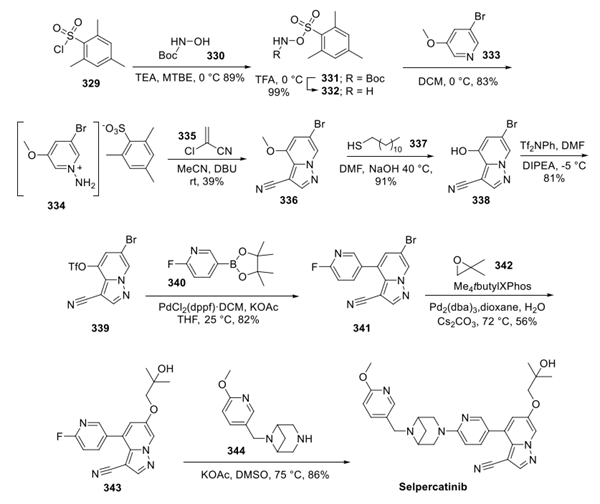
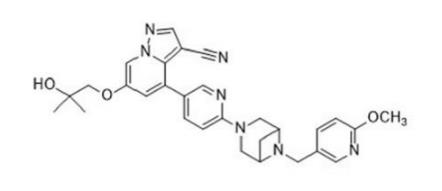
Selpercatinib (LOXO-292):Indications, Mechnaism,Clinical trials and Side effects
Selpercatinib (LOXO-292) is a potent, adenosine triphosphate–competitive, highly selective small molecule RET inhibitor with nanomolar potency against diverse RET alterations (including anticipated acquired gatekeeper resistance mutations).

Mechnaism of action
LOXO-292 is an orally administered selective RET (REarranged during Transfection) tyrosine kinase inhibitor. The RET kinase protein, which is encoded by the RET proto-oncogene, is involved in cell signaling, proliferation, and differentiation. RET gene fusions or mutations can lead to ligand-independent constitutive activation of the RET kinase signaling pathway. Selpercatinib binds to the ATP-binding site and locks the kinase in the inactive state, preventing downstream signaling and cell proliferation.
Indications
Selpercatinib (LOXO-292) is a highly selective RET inhibitor with activity across known oncogenic RET fusions and mutations. Based on initial data from LIBRETTO-001, LOXO-292 received FDA Breakthrough Designation for the treatment of RET fusion+ non-small cell lung cancer (NSCLC), RET fusion+ thyroid cancer and RET-mutant medullary thyroid cancer (MTC).
Clinical trials
Selpercatinib (LOXO-292) has preclinical nanomolar potency against diverse RET alterations (e.g. fusions, activating mutations and anticipated acquired resistance mutations) and anti-tumor activity in the brain. LOXO-292 has demonstrated clinical activity in adult patients with RET-alterated solid tumors.
Methods: LIBRETTO-121 (EudraCT 2019-000212-28) is an ongoing multicenter phase 1/2 dose escalation multicenter trial in patients 6 months-21 years of age with advanced, RET-altered solid and CNS tumors. Dose escalation follows a rolling 6 design starting at the equivalent of the adult recommended phase 2 dose. Enrollment began on 12 Feb 2019 and is ongoing. Key eligibility criteria include: solid or CNS tumor with a documented RET gene alteration refractory to standard therapy; age 6 months to 21 years of age; and adequate bone marrow, liver and kidney function. LOXO-292 is administered orally BID for continuous 28-day cycles. Both capsule and liquid suspension dosage forms are available. The primary objective of the phase 1 portion of the study is to determine safety and dose limiting toxicities. Key secondary objectives include characterization of pharmacokinetic properties, identification of the MTD and initial characterization of the anti-tumor activity of LOXO-292. Archival tissue will be used to further characterize molecular abnormalities.[1]
Side effects
The most common adverse reactions (≥25%) of Selpercatinib (LOXO-292) were edema, diarrhea, fatigue, dry mouth, hypertension, abdominal pain, constipation, rash, nausea, and headache. The most common Grade 3 or 4 laboratory abnormalities (≥5%) were decreased lymphocytes, increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), decreased sodium, and decreased calcium.
Selpercatinib(LOXO-292) can cause serious side effects including liver toxicity, high blood pressure, heart rhythm changes due to prolongation of heart electrical activity (QT prolongation), bleeding, allergic reactions, impaired wound healing and harm to an unborn baby.
Reference
[1] S. DuBois. “A phase I study of LOXO-292, a highly selective RET inhibitor, in pediatric patients with RET-altered cancers.”Journal of Clinical Oncology (2019).
Related articles And Qustion
Lastest Price from LOXO-292 manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 2152628-33-4
- Min. Order:
- 1kg
- Purity:
- >99.5% by HPLC
- Supply Ability:
- 100kg/month
US $2.00/kg2025-04-21
- CAS:
- 2152628-33-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 30KG/M