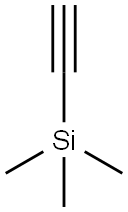
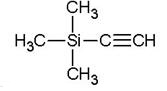
Ethynyltrimethylsilane: the bright star of chemical synthesis and materials science
Abstract
This paper reviews the advanced applications and potential value of Ethynyltrimethylsilane in the field of chemical synthesis and materials science. Firstly, the basic physical and chemical properties, synthesis methods, and their importance in modern science are briefly introduced. Then, from the aspects of organic synthesis, drug research and development, materials science, etc., the application examples and advantages are described in detail. Finally, the future development prospects, the challenges, and possible solutions are discussed.
Introduction
In the vast field of chemical synthesis and materials science, Ethynyltrimethylsilane has gradually emerged and become a bright new star with its unique chemical properties and wide application prospects. As an important member of organosilicon compounds, it not only has excellent chemical stability and reactivity but also shows great potential in many fields such as organic synthesis, drug research and development, materials science, and other fields. In this paper, the basic properties, synthesis methods, application fields, and future development of these compounds will be reviewed and analyzed.
Basic properties and synthesis methods
Basic property
Ethynyltrimethylsilane silicon, chemical formula C5H10Si, molecular weight 98.22. It is a colorless, transparent liquid with a high boiling point (53°C) and a low melting point (>0°C). The property is stable at room temperature and pressure, but it is easy to oxidize in the air and the color is deepened. In addition, it is sensitive to water and acid, so it is necessary to avoid contact with water and acid during storage and use.
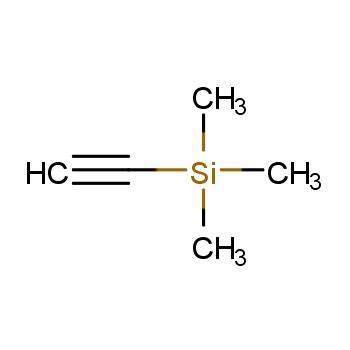
Synthesis method
The synthesis methods of ethynyltrimethylsilane silicon mainly include direct synthesis and indirect synthesis. The direct synthesis method is usually produced by the reaction of trimethylchlorosilane and acetylene under the action of a catalyst. Indirect synthesis is the reaction of other organosilicon compounds with acetylene or other alkynes. Both methods have advantages and disadvantages, and the specific choice depends on factors such as raw material source, reaction conditions, and product purity.1
Chemical synthesis applications
Important reagents for organic synthesis
As an important organic synthesis reagent, ethynyltrimethylsilane silicon has been widely used in the field of organic synthesis. It can react with a variety of organic compounds, such as addition reaction, substitution reaction, coupling reaction, etc., to generate organic compounds with specific structure and function. For example, it can be added with aldehydes, ketones, and other carbonyl compounds to generate the corresponding enols or ketenes; It can also be substituted with halogenated hydrocarbons to produce silicone compounds with specific substituents. These reactions not only enrich the types and structures of organic compounds but also provide an important material basis for the research and development of new drugs, materials science, and other fields.2
Key intermediates in drug discovery
In the field of drug development, ethynyltrimethylsilane silicon is also of great value. It can be used as a key intermediate in drug synthesis, participate in the construction and modification of drug molecules, and can change the physical and chemical properties of drug molecules, such as solubility, stability, biological activity, etc., to improve the efficacy of drugs and reduce side effects. In addition, it can also be used as the carrier of a drug delivery system to effectively transport drug molecules to target tissues or cells to achieve precise treatment of drugs.3
Application of ethynyltrimethylsilane silicon in materials science
Preparation and modification of silicon-based materials
Ethynyltrimethylsilane silicon plays an important role in the preparation and modification of silicon-based materials. It can be used as a precursor or modifier of silicon-based materials to prepare silicon-based materials with specific structures and properties by reacting or copolymerizing with other silicon-based compounds. For example, condensation reaction with silane, silanol, and other compounds to produce siloxane or silanol polymer; It can also be surface modified with nanoparticles to prepare silicon-based nanocomposites with specific surface properties and functions. These silicon-based materials have wide application prospects in electronic devices, optical devices, biosensors, and other fields.4
Synthesis of organic-inorganic hybrid materials
ethynyltrimethylsilane silicon can also be used as one of the synthetic raw materials of organic-inorganic hybrid materials. Through copolymerization or grafting with organic polymers, organic-inorganic hybrid materials with excellent properties can be prepared. These materials not only have the flexibility, processability, and functionality of organic polymers but also have the stability, high-temperature resistance, and corrosion resistance of inorganic materials. Therefore, it has a wide range of application prospects in aerospace, automobile manufacturing, electronic information, and other fields.5
Future development and prospects
Ethynyltrimethylsilane silicon has broad application prospects and potential in organic synthesis, materials science, and other fields. In the future, with the continuous development of science and technology and the improvement of environmental awareness, it will play an important role in more fields and make greater contributions to the sustainable development of human society.
References
[1].Seyferth, D.; Vick, S. C., Organolithium routes to 1, 2-disubstituted ethylene derivatives. An attempted synthesis of 1, 2-dilithioethylene. Journal of Organometallic Chemistry 1978, 144 (1), 1-12.
[2]. Austin, W. B.; Bilow, N.; Kelleghan, W. J.; Lau, K. S., Facile synthesis of ethynylated benzoic acid derivatives and aromatic compounds via ethynyltrimethylsilane. The Journal of Organic Chemistry 1981, 46 (11), 2280-2286.
[3].Havens, S. J.; Hergenrother, P. M., Synthesis of arylacetylenes by the sodium hydride catalyzed cleavage of 4-aryl-2-methyl-3-butyn-2-ols. The Journal of Organic Chemistry 1985, 50 (10), 1763-1765.
[4].Grirrane, A.; álvarez, E.; García, H.; Corma, A., Preparation of Tremorine and Gemini Surfactant Precursors with Cationic Ethynyl‐Bridged Digold Catalysts. Chemistry–A European Journal 2017, 23 (12), 2792-2801.
[5]. Sabourin, E. T.; Onopchenko, A., A convenient synthesis of 4-ethynylphthalic anhydride via 2-methyl-3-butyn-2-ol. The Journal of Organic Chemistry 1983, 48 (25), 5135-5137.
You may like
Related articles And Qustion
Lastest Price from Trimethylsilylacetylene manufacturers

US $10.00/KG2025-04-21
- CAS:
- 1066-54-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $0.00-0.00/kg2025-04-18
- CAS:
- 1066-54-2
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 97%
- Supply Ability:
- 50tons