The Efficacy and Applications of N-Chlorosuccinimide: A Comprehensive Insight
Introduction
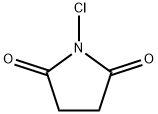
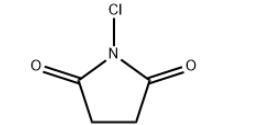
N-Chlorosuccinimide (1-chloropyrrolidine-2,5-dione;NCS) was first synthesized in 1886 by the chlorination of succinimide with chlorinated lime. In newer methods,potassium hypochlorite, tert-butylhypochlorite and chlorine in aqueous sodium hydroxide have been applied for this transformation. From the very beginning, NCS was used in chlorinationand oxidation reactions. In the last 20 years, however,there has been an upsurge of interest in NCS, arising fromthe observation that it is a versatile reagent capable of me-diating a plethora of different chemical transformations.
Chemical property
N-chlorosuccinimide, C4H4C1N02, was made from succinimide by the method of Hirst & Macbeth (1922). It is a colourless crystalline solid melting at 148 °C. Crystals were obtained from benzene by slow evaporation as small orthorhombic plates tabular on {001 } with the additional forms {111 } (always present), {110} (common), {011} (rare); {101 } (very rare)[1].

N-chlorosuceinimide forms orthorhombic crystals with a=6.41, b = 7.11, c = 11.69Å, space group P212121 and Z =4. The structure has been solved by application of the heavy-atom method to the bromine isomorph and refined by two-dimensional projections on the (100) and (010) planes. The molecular bond lengths and angles are bilaterally symmetrical, but the molecule is significantly aplanar. A 'close' intermolecular approach of 2.88 Å has been observed between C1 and O.
Uses
N-Chlorosuccinimide (NCS) is a five-membered N-containing heterocyclic molecule. NCS is a versatile reagent and itssignificance is not limited to chlorination and oxidation. It mediatesor catalyzes many chemical reactions, including halocyclizations,formation of heterocyclic systems, formation of new carbon–carbonbonds, rearrangements, and functional group transformations. NCS is slowly hydrolyzed by water(pK 5.82) with formation of hypochlorous acid which is asource of bactericidal activity. Its reaction with a hydro-halic acid affords succinimide and the halogen. The oxi-dation of halides to halogens is used in the test withiodide-starch paper to indicate the presence of NCS in areaction mixture. NCS is soluble in CCl4 at room temper-ature, whereas its conjugate product, succinimide, is not;this facilitates the separation of the reaction mixture. Justlike the other N-chloroamines, NCS is, to some extent,thermally unstable and there have been reports of violentreactions with alcohols and benzylamine.NCS is one ofseveral commercially available N-chloramines: chlor-amine-T, 1,3-dichloro-5,5-dimethylhydantoin (NDDH), trichloroisocyanuric acid (TCCA)[2].
References:
[1] BROWN R N. The crystal structure of N‐chlorosuccinimide[J]. Acta Crystallographica, 1961, 78 1: 1099-1100. DOI:10.1107/S0365110X61002175.[2] W. GO??BIEWSKI M G. Applications of N-Chlorosuccinimide in Organic Synthesis[J]. ChemInform, 2007, 25 1. DOI:10.1002/CHIN.200810244.
Related articles And Qustion
Lastest Price from N-Chlorosuccinimide manufacturers

US $0.00/G/KG2025-04-22
- CAS:
- 128-09-6
- Min. Order:
- 1G/KG
- Purity:
- 99%
- Supply Ability:
- 100MT

US $10.00/KG2025-04-21
- CAS:
- 128-09-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt