Trimethylsilylacetylene-Uses & Properties
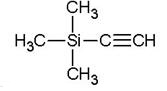
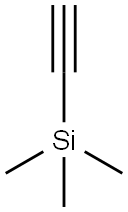
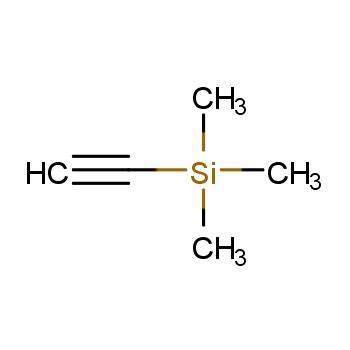
Trimethylsilylacetylene is the alkynyl trialkylsilanes, also named tms acetylene. It is an important spice and pharmaceutical intermediate. As a source of HC≡C−, trimethylsilylacetylene was widely used in alkynylation. In the preparation of various monocylenes, polyselenes, trimethylsilylacetylene was essential synthetic primitives. The protected group, trimethylsilyl, can be desilyated with TBAF expediently. Using this protected alkyne, as opposed to acetylene itself, further coupling reactions could be prevent and also has the benefit of being a liquid.[1] In the other hand, trimethylsilylacetylene was also employed as raw materials of silicatization reagent. In addition, there are many applications of trimethylsilylacetylene in heterocyclic chemistry, carbon-carbon coupling, drug synthesis, catalytic chemistry and materials industries.
What is more, being a silicon-based protective group, trimethylsilylacetylene could be used for the synthesis of pyrazole by the addition of 1, 3-bipolar ring to acetylene diazo compounds. As well, it acted as the substrate for coupling reaction with benzyl nitrile under the meditation of nickel.
Trimethylsilylacetylene is commercially available. It may also be prepared in a manner similar to other silyl compounds. Deprotonation of acetylene with a Grignard reagent was taken place firstly. Then, trimethylsilyl chloride was dropped and stirred. With the reaction, trimethylsilylacetylene was obtained. [2] By the way, a less expensive alternative reagent is 2-methylbut-3-yn-2-ol, which after alkynylation could be deprotected with base.
Employing trimethylsilylacetylene, an efficient synthetic route to (E)-1,3-bissilyl-6-arylfulvenes has been developed. In the presence of a catalytic amount of PdBr2, with the reaction between aryl iodides and trimethylsilylacetylene, good to excellent yields of 6-aryl-1,3-bis(trimethylsilyl)fulvenes was obtained with complete regio- and stereoselectivity. The reaction involves trimerization of trimethylsilylacetylene and cleavage of one silyl group. The silylated fulvenes obtained could be converted into halogenated fulvenes by site-selective halodesilylation. The halogenated fulvenes underwent the Stille coupling leading to the corresponding arylated fulvenes.
A comparative study on CoIII-catalyzed direct C-H bond alkenylation of 1-phenylpyrazole derivatives with alkynyl carboxylic acids and arylalkynylsilanes under redox‐neutral conditions was disclosed by Suzuki and co-workers. These methods show excellent selectivity with good to excellent yields. Trimethylsilylacetylene has been utilized as a vinyl source to obtain the corresponding styrene derivatives. The major differences between these two reactions are the decarboxylation proceeds in the presence of a base additive whereas the desilylation takes place in the presence of an acid additive. The developed methodologies are compatible with a variety of functional groups.
References
[1] Godson, C.; Nwokogu; Zemolka, S.; Dehmel, F. (2007). Trimethylsilylacetylene. doi: 10.1002/047084289X.rt288.pub2.
[2] Andrew, B.; Holmes; Chris, N.; Sporikou (1993). Trimethylsilylacetylene. Organic Syntheses.; 8, 606.
[3] Kumar, A.; Muniraj, N.; Prabhu, K. R. Cobalt(III)‐Catalyzed Direct ortho‐Alkenylation of Arylpyrazoles: A Comparative Study on Decarboxylation and Desilylation, Europ. J. Org. Chem., 2019, 16, 2735-2739.
[4] Suzuki, S.; Kinoshita, H.; Miura, K. Palladium-Catalyzed Regio- and Stereoselective Synthesis of (E)-1,3-Bissilyl-6-arylfulvenes from Aryl Iodides and Silylacetylenes, Org. Lett., 2019, 21(6): 1612-1616.
You may like
Related articles And Qustion
See also
Lastest Price from Trimethylsilylacetylene manufacturers

US $10.00/KG2025-04-21
- CAS:
- 1066-54-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $0.00-0.00/kg2025-04-18
- CAS:
- 1066-54-2
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 97%
- Supply Ability:
- 50tons