Ethyl propiolate: A Versatile Compound with Applications in Pyrazole Synthesis and Gasoline Additives
General Description
Ethyl propiolate, a colorless liquid with a fruity odor, is widely utilized in the food, pharmaceutical, and cosmetic industries. Its synthesis involves esterification or transesterification reactions. Furthermore, it serves as a crucial reactant in dipolar cycloaddition reactions for pyrazole synthesis, catalyzed by transition metal catalysts. The resulting pyrazoles have diverse biological activities and are valuable in medicinal and agrochemical applications. Additionally, Ethyl propiolate is employed as a gasoline additive to enhance combustion performance, as demonstrated by its effects on critical properties when combined with various gasoline components.

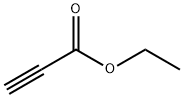
Figure 1. Ethyl propiolate
Synthesis
Ethyl propiolate, also known as ethyl propanoate, is a colorless liquid with a fruity odor. Its chemical formula is C5H6O2 and it is commonly used in the food and beverage industry as a flavoring agent, as well as in the pharmaceutical and cosmetic industries as a solvent. Ethyl propiolate can be synthesized through the esterification reaction between ethanol and propionic acid, which is a type of condensation reaction that forms an ester and water. The reaction is typically carried out under acidic conditions, with the addition of a catalyst such as sulfuric acid or p-toluenesulfonic acid. The resulting mixture is then distilled to isolate the Ethyl propiolate product. Alternatively, Ethyl propiolate can also be obtained through the transesterification reaction between ethyl alcohol and propyl acetate, which is a process that involves the exchange of ester groups between two different molecules. This method is less commonly used but may offer advantages in certain situations. Overall, Ethyl propiolate is a versatile compound with a wide range of applications due to its pleasant aroma and solubility properties. Its reliable synthesis methods make it a valuable ingredient in various industries. 1
Application in the Synthesis of Pyrazoles
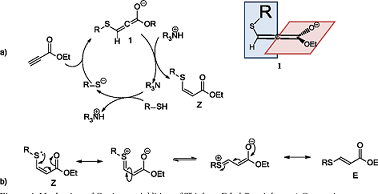
Ethyl propiolate is an organic compound commonly used in dipolar cycloaddition reactions to form heterocycles, particularly pyrazoles. Dipolar cycloaddition reactions involve the combination of a dipolar species, such as a nitrile imine or diazo compound, with an electron-rich π-system, such as an alkene or alkyne, to form a new cyclic structure. In the context of pyrazole synthesis, Ethyl propiolate serves as an important reactant. It can undergo a dipolar cycloaddition reaction with various dipolarophiles, such as diazo compounds or nitrile imines, to yield pyrazoles. This reaction is often catalyzed by transition metal catalysts, such as copper or silver, which facilitate the formation of the new carbon-carbon bonds. The resulting pyrazoles are versatile heterocyclic compounds widely used in medicinal chemistry and agrochemical industries. They possess a unique structure and exhibit diverse biological activities, including anti-inflammatory, antifungal, and anticancer properties. Therefore, the development of efficient methods for synthesizing pyrazoles using Ethyl propiolate as a starting material has garnered significant attention in organic synthesis. In summary, Ethyl propiolate finds applications in dipolar cycloaddition reactions leading to the synthesis of heterocycles, specifically pyrazoles. These compounds have a wide range of potential pharmaceutical and agricultural uses due to their diverse biological activities. 2
Application in Gasoline Additives
Ethyl propiolate is commonly used as an additive to enhance the combustion performance of gasoline. In a study, the critical temperature and critical pressure of several binary and one ternary system containing Ethyl propiolate were measured. The five binary systems studied were ethyl propionate + n-hexane, ethyl propionate + n-heptane, ethyl propionate + n-octane, ethyl propionate + n-propanol, and ethyl propionate + n-butanol. The ternary system studied was ethyl propionate + n-hexane + n-propanol. The results indicated that the addition of ethyl propionate increased the Tc of n-hexane, n-propanol, and n-heptane, while decreasing the Tc of n-octane and n-butanol. Moreover, the binary system of ethyl propionate and n-heptane, as well as the ternary system of ethyl propionate + n-hexane + n-propanol, exhibited positive azeotropes. Furthermore, the pc values of all systems studied showed a linear dependence on the concentration of Ethyl propiolate. The applicability of Redlich-Kister equations, Cibulka equations, and Singh-Sharma equations was assessed to ensure accurate predictions and analysis of the obtained data. Overall, this research provides valuable insights into the critical properties of Ethyl propiolate when combined with various gasoline components, contributing to a better understanding of its potential as an additive for improved combustion performance. 3
Reference
1. Ethyl propiolate. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 12182.
2. Liu Y, Zhao Q, Wang H. Design strategy research for synthesis of ethyl propionate and n-propyl propionate by reactive distillation. Computers & Chemical Engineering, 2024, 12: 1.
3. Tian L. Measurements of critical properties of ethyl propionate with the gasoline components. Journal of Molecular Liquids, 2021, 322(15): 114801.
Related articles And Qustion
Lastest Price from Ethyl propiolate manufacturers
US $0.00-0.00/kg2025-04-04
- CAS:
- 623-47-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton

US $0.00/KG2025-03-07
- CAS:
- 623-47-2
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 5MT/Month