Dilauroyl Peroxide: A Versatile Compound in Polymerization with Significant Safety Considerations
General Description
Dilauroyl peroxide is a white, stable solid with a molecular weight of 398.62 and a melting point range of 53 to 55°C. It decomposes at 62°C and is soluble in organic solvents but insoluble in water. As a chemical initiator in polymerization and a cross-linking agent, it plays a crucial role in various industrial applications. The synthesis process involves the reaction of lauroyl chloride with hydrogen peroxide, resulting in a high yield and purity. However, dilauroyl peroxide poses significant safety hazards, being highly flammable, sensitive to shock, and prone to explosion when heated. Proper handling and safety precautions are essential to prevent accidents and ensure personal and environmental safety.

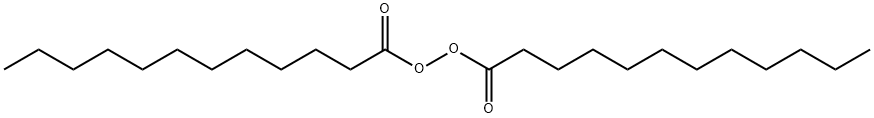
Figure 1. Dilauroyl peroxide
Characteristics
Dilauroyl peroxide is a white granular solid with a molecular weight of 398.62. It has a melting point range of 53 to 55°C and decomposes at 62°C. The compound exhibits a 4.02% active oxygen content and requires an activation energy of 128.54 kJ/mol for its chemical processes. Dilauroyl peroxide is soluble in organic solvents such as acetone and chloroform but insoluble in water. It remains stable at room temperature; however, it is prone to explosion when heated. Importantly, it is non-toxic. These properties make dilauroyl peroxide valuable for various applications. Its controlled decomposition at a relatively low temperature makes it useful as a chemical initiator in polymerization reactions, where its high purity and stability are essential to ensure the quality of the final product. Additionally, its solubility in organic solvents enables its use as a cross-linking agent in certain polymer systems. However, caution must be exercised due to its potential explosive nature when exposed to heat. 1
Synthesis
Dilauroyl peroxide is synthesized through the reaction of lauroyl chloride with hydrogen peroxide. The process involves dissolving lauroyl chloride in petroleum ether, followed by the addition of sodium hydroxide. Subsequently, 6% hydrogen peroxide is introduced for oxidation. As the temperature reaches 45°C, the reaction mixture is vigorously stirred and then cooled by adding ice water. Crystallization occurs, and the resulting crystals are filtered with stirring, washed to neutrality, and then air-dried to obtain the final product. This synthetic method is widely employed due to its relative simplicity and efficiency in producing dilauroyl peroxide. The use of petroleum ether as a solvent and the controlled addition of reactants contribute to the successful formation of the compound. Additionally, the cooling and filtration steps aid in obtaining a purified product. Overall, this synthesis route allows for the production of dilauroyl peroxide with high yield and purity, making it accessible for various industrial and research applications. 2
Safety
Dilauroyl peroxide poses significant safety hazards and must be handled with extreme caution. Contact with the liquid can cause irritation to the eyes and skin, while ingestion may lead to irritation of the mouth and stomach. The substance is highly flammable and can increase the severity of a fire. Additionally, it becomes sensitive to shock when hot, and containers may explode in a fire. Dilauroyl peroxide may also ignite or explode spontaneously if mixed with flammable materials. As an organic peroxide, it contains sufficient available oxygen to support its own combustion, even in the absence of atmospheric oxygen. Furthermore, it is a strong oxidizing material. In case of a fire outbreak, fire precautions should be taken, such as spraying water hoses from areas as far as possible and free from possible explosion risk, and spraying abundant water over the containers to protect them from overheating. Due to its explosive potential, separation of shock-sensitive peroxides from their safety diluent can create a hazardous condition. It is crucial for individuals handling dilauroyl peroxide to be aware of these hazards and to take appropriate safety measures to prevent accidents and ensure personal and environmental safety. 3
Reference
1. Lauroyl peroxide. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 7773.
2. Lower ES. Lauroyl peroxides as polymer catalysts and other uses. Pigment & Resin Technology, 1980, 9(10): 11-12.
3. PubChem Annotation Record for DILAUROYL PEROXIDE. National Center for Biotechnology Information (2024). Source: Hazardous Substances Data Bank.
Related articles And Qustion
See also
Lastest Price from Lauroyl peroxide manufacturers

US $10.00-8.00/kg2025-04-21
- CAS:
- 105-74-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $100.00-4000.00/kg2025-04-17
- CAS:
- 105-74-8
- Min. Order:
- 1000kg
- Purity:
- 0.98
- Supply Ability:
- 100 Metric Ton/Metric Tons per Year