Lauroyl peroxide: A widely used organic substance
Description
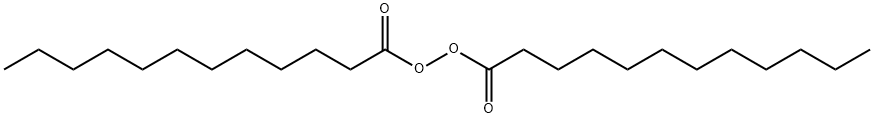
Lauroyl peroxide is an organic compound with the formula (C11H23CO2)2. A colorless solid, it is often sold as a water-damped solid. It is the symmetrical peroxide of lauric acid. The product of commerce is a soft, white flowing granular waxy material with a slight odour and little taste. Its molecular weight is 398.63 and the melting point is 54/55 ℃。 It is insoluble in water and slightly soluble in alcohol. It can be dried in the form of an emulsion by centrifuging.
Preparation
There are many ways to synthesize Lauroyl peroxide. It can be prepared by the reaction of lauroyl chloride with hydrogen alkali metal peroxides. Such as the treatment of lauroyl chloride with hydrogen peroxide and potassium hydroxide could prepare Lauroyl peroxide. It has also been prepared by reacting a mixture of lauroyl chloride, lauric anhydride, and phosphorous trichloride in benzene, with a mixture of hydrogen peroxide, water, sodium phosphate, and caustic soda[1].
Uses
Lauroyl peroxide could used as a catalyst for the polymerization of vinyl monomers and is particularly useful for the suspension polymerization of vinyl chloride. In conjunction with amine activators, it can also be employed in the polymerization of unsaturated polyesters and methyl methacrylate resins[2]. Lauroyl peroxide has been exploited as a catalyst in the preparation of vinyl fluorene, and the making of vinyl fluoride copolymer laminates.
Lauroyl peroxide could used with ferrocine as a hardening agent and accelerator for epoxy resins. It has been used as a catalyst in the production of epoxide acrylate grafts of thermoplastic polymers to give polymers of low ketone solubility and improved heat stability; and in a catalyst system for preparing ethyl hexyl polymers for grafting with vinyl chloride to give impact-resistant copolymers. It has also been put to use in making ethylene-based graft polymers. As a cosurfactant, Lauroyl Peroxide could used in miniemulsion Polymerization[3].
References
[1] Lower E, et al. Lauroyl peroxides as polymer catalysts and other uses. Pigment Resin Technology, 1980; 9: 11-12.
[2] Bevington J, et al.The use of stabilized radicals with monomers and lauroyl peroxide. European Polymer Journal, 2004; 40: 103-108.
[3] Reimers J, et al. Lauroyl Peroxide as a Cosurfactant in Miniemulsion Polymerization. Industrial Engineering Chemistry Research, 1997; 36: 1085–1087.
You may like
Related articles And Qustion
See also
Lastest Price from Lauroyl peroxide manufacturers

US $10.00-8.00/kg2025-04-21
- CAS:
- 105-74-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $100.00-4000.00/kg2025-04-17
- CAS:
- 105-74-8
- Min. Order:
- 1000kg
- Purity:
- 0.98
- Supply Ability:
- 100 Metric Ton/Metric Tons per Year