Ethyl propiolate: properties, applications as analytical reagent and safety
General Description
Ethyl propiolate is a colorless liquid with a fruity odor commonly used as a versatile derivatizing agent for thiols. It reacts readily with thiols to form disulfides, enabling their modification and characterization in chemical and biological research. Ethyl propiolate is also applied as an analytical reagent for thiols, offering a new approach in pharmaceutical analysis and thiol determination. In terms of safety, it has a low toxicity profile, and exposure at normal levels does not cause adverse health effects. However, high concentrations may lead to irritation, requiring proper handling and ventilation. Ethyl propiolate is considered safe when used according to established guidelines.

Figure 1. Ethyl propiolate
Properties
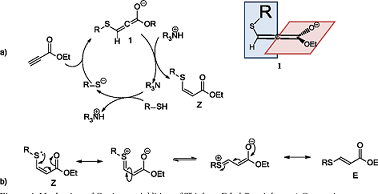
Ethyl propiolate is an organic compound with the chemical formula C5H6O2. It is a colorless liquid with a fruity odor and is commonly used as a reagent in various chemical reactions. One of the notable properties of ethyl propiolate is its ability to act as a versatile derivatizing agent for thiols or sulfhydryl compounds. Thiols are organic compounds containing a sulfur atom bonded to a hydrogen atom, and they play important roles in various biological and chemical processes. Ethyl propiolate reacts readily with thiols to form disulfides through a nucleophilic addition-elimination reaction. This reaction is often used in organic synthesis to modify and functionalize thiols. The resulting disulfides can be further manipulated to introduce desired functionalities or to create new chemical entities. The use of ethyl propiolate as a derivatizing agent provides researchers with a valuable tool to study thiols and their functions in various systems. It allows for the modification of thiol-containing molecules and facilitates their detection and characterization using analytical techniques such as chromatography and spectroscopy. In summary, ethyl propiolate exhibits the property of reactivity towards thiols, making it a useful reagent for derivatizing sulfhydryl compounds and enabling their study and manipulation in chemical and biological research. 1
Applications as analytical reagent
Ethyl propiolate is a novel derivatizing reagent with various applications in analytical chemistry. This introduction highlights its potential as an analytical reagent, particularly for thiols. The present study represents the first systematic investigation of EP in analytical chemistry, specifically in relation to its use as a derivatizing reagent for thiols. The reaction was studied under flow conditions using sequential injection (SI) analysis and UV detection at 285 nm. Several parameters such as pH, concentration of EP, and temperature were found to affect the reaction kinetics, which were thoroughly examined through stopped-flow experiments. Cysteine (CYS) and captopril (CAP) were chosen as model thiolic compounds due to their similar chemical structures. The researchers successfully demonstrated the applicability of EP as a derivatization reagent by developing and validating an automated assay for the determination of CAP in pharmaceuticals. Various parameters of the post-column reaction were investigated, including pH, amount concentration of the reagent, flow rates, length of the reaction coil, and temperature. The linear determination range for GSH was found to be 1-200 mol/L, with a limit of detection (LOD) of 0.1 mol/L (S/N = 3). Overall, these findings demonstrate that ethyl propiolate holds great potential as an analytical reagent for thiol compounds, offering a new approach in analytical chemistry for applications such as pharmaceutical analysis and determination of thiols in complex matrices like vegetable samples. 2
Safety
Ethyl propionate is a chemical compound used in various industries as a solvent and fragrance ingredient. In terms of safety, ethyl propionate has been extensively studied and is considered to be safe for use. The substance has a low toxicity profile, and exposure to it at normal levels does not cause adverse health effects. When ingested, ethyl propionate is rapidly absorbed, metabolized, and eliminated from the body through urine and breath. In animal studies, high doses of ethyl propionate have not shown any signs of toxicity, indicating that it is unlikely to pose any significant risk to humans under normal conditions of use. However, as with any chemical substance, exposure to high concentrations of ethyl propionate may cause irritation or other adverse effects. It is important to handle this substance with care and ensure proper ventilation and personal protective equipment when working with it. Overall, when used according to established safety guidelines, ethyl propionate is considered to be a safe ingredient in various applications and does not pose a significant risk to human health. 3
Reference
1. Poulsen TB, Bernardi L, Aleman J, Overgaard J, J?rgensen KA. Organocatalytic asymmetric direct alpha-alkynylation of cyclic beta-ketoesters. J Am Chem Soc. 2007;129(2):441-449.
2. Zacharis CK, Tzanavaras PD, Zotou A. Ethyl propiolate as a post-column derivatization reagent for thiols: development of a green liquid chromatographic method for the determination of glutathione in vegetables. Anal Chim Acta. 2011;690(1):122-128.
3. Api AM, Belsito D, Botelho D, et al. RIFM fragrance ingredient safety assessment, ethyl propionate, CAS Registry Number 105-37-3. Food Chem Toxicol. 2022;167 Suppl 1:113343.
Related articles And Qustion
See also
Lastest Price from Ethyl propiolate manufacturers
US $0.00-0.00/kg2025-04-04
- CAS:
- 623-47-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton

US $0.00/KG2025-03-07
- CAS:
- 623-47-2
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 5MT/Month