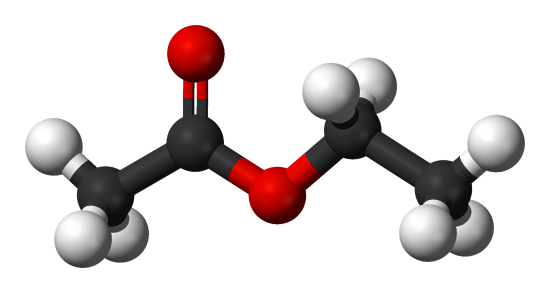
Ethyl acetate - Uses, Properties, Safety etc.
Ethyl acetate, also known as ethyl ethanoate, is an important and widely used chemical in industry. It has an important role as a solvent in adhesives, paints, or coatings, and it can be used to substitute aromatic solvents such as toluene. Additionally, ethyl ethanoate exhibits similar physicochemical properties as some of previously discussed chemicals which may show potential as diesel additives.

Production and Synthesis
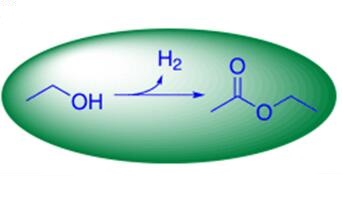
In fact, there are several ethyl acetate production technology alternatives. Traditionally, ethyl acetate has been manufactured from fossil-based resources but in terms of sustainability, recent research efforts have focused in the investigation of a possibility of a simple and economical one-pot synthesis strategy, taking advantage of bioethanol. The main fossil-based process comprises the esterification reaction between ethanol and acetic acid. In this process, ethanol is produced by hydration of ethylene and acetic acid by methanol carbonylation reaction. The process is known to be acid catalyzed and liquid sulfuric acid is a commonly used in the reaction. As mentioned earlier, liquid acids are not anymore suitable due to tightening environmental requirements and difficult handling problems. As a result, some researches have demonstrated the process over solid acid catalysts such as zeolites, aluminum sulfates, and heteropolyacid-supported montmorillonites. Additionally, some other ways to produce ethyl ethanoate comprise the so-called Tishchenko reaction between two acetaldehyde molecules, liquid-phase oxidation of n-butane, copolymerization of butyraldehyde and polyvinyl acetate, and esterification of acetic acid and ethylene.
The simple ethanol and aqueous ethanol-based process to ethyl acetate is the most important approach today. It is also worth mentioning that the possibility to carry out the reaction without any additional reagents and nonfossil-based feedstocks is a clear benefit.
Uses
Ethyl acetate is used primarily as a solvent and diluent, being favored because of its low cost, low toxicity, and agreeable odor.For example, it is commonly used to clean circuit boards and in some nail varnish removers (acetone is also used). Coffee beans and tea leaves are decaffeinated with this solvent (when supercritical CO2 extraction is not possible). It is also used in paints as an activator or hardener. Ethyl acetate is present in confectionery, perfumes, and fruits. In perfumes, it evaporates quickly, leaving only the scent of the perfume on the skin.
Ethyl acetate is used as a solvent for varnishes, lacquers, and nitrocellulose; as anartificial fruit flavor; in cleaning textiles;and in the manufacture of artificial silk andleather, perfumes, and photographic filmsand plates. Ethyl Acetate is generally used as a solvent in organic reactions. Environmental contaminants; Food contaminants.
Reactions
Ethyl acetate can be hydrolyzed in acidic or basic conditions to regain acetic acid and ethanol. The use of an acid catalyst accelerates the hydrolysis, which is subject to the Fischer equilibrium mentioned above. In the laboratory, and usually for illustrative purposes only, ethyl esters are typically hydrolyzed in a two step process starting with a stoichiometric amount of strong base, such as sodium hydroxide. This reaction gives ethanol and sodium acetate, which is unreactive toward ethanol:
CH3CO2C2H5 + NaOH → C2H5OH + CH3CO2Na
Safety Information
Potentially poisonous by ingestion. Toxicity depends upon alcohols in question, generally ethanol with methanol as a denaturant. A flammable liquid and dangerous fire hazard; can react vigorously with oxidzing materials. Moderate explosion hazard.
You may like
Related articles And Qustion
Lastest Price from Ethyl acetate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 141-78-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $0.00/kg2025-04-15
- CAS:
- 141-78-6
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 20 tons