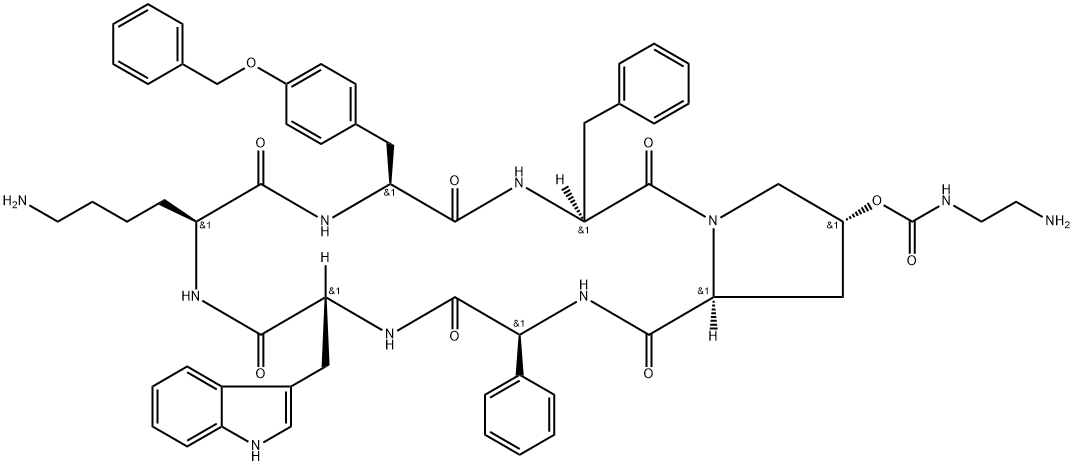
A synthetic polypeptide drug:Pasireotide
General description
Pasireotide is a synthetic polypeptide analogue of somatostatin that resembles the native hormone in its ability to suppress levels and activity of growth hormone, insulin, glucagon and many other gastrointestinal peptides. Because its half-life is longer than somatostatin, pasireotide can be used clinically to treat neuroendocrine pituitary tumors that secrete excessive amounts of growth hormone causing acromegaly, or adrenocorticotropic hormone (ACTH) causing Cushing disease. Pasireotide has many side effects including suppression of gall bladder contractility and bile production, and maintenance therapy can cause cholelithiasis and accompanying elevations in serum enzymes and bilirubin.
A somatostatin analogue with pharmacologic properties mimicking those of the natural hormone somatostatin; used (as its diaspartate salt) for treatment of Cushing's disease. It has a role as an antineoplastic agent. It is a homodetic cyclic peptide and a peptide hormone. It is a conjugate base of a pasireotide(2+).[1]
Application and pharmacology
1.Pasireotide is a novel multireceptor-targeted somatostatin analog with high affinity for SSTR1, 2, 3, and 5. Pasireotide is a second generation multireceptor-targeted somatostatin analog with a broader spectrum of affinity with respect to octreotide and lanreotide for different receptor subtypes (SSTR1, 2, 3, 5). Pasireotide is currently approved for the treatment of acromegaly and Cushing's disease. This paper provides a state-of-the-art overview of pasireotide in the treatment of NETs, with the aim of addressing clinical relevance and future perspectives for this molecule in the management of NETs. Pasireotide expands therapeutic options improving biochemical and clinical outcomes. This novel agent seems to be the effective treatment option in patients with acromegaly who do not respond to the first-line SSAs. Pasireotide LAR should be started at a dose of 40 mg given i.m. every 28 days, and if there is no response, the initial dose should be increased to 60 mg i.m. every 28 days. Moreover, in Cushing's patients the therapy with pasireotide might reduce signs and symptoms and improve QoL, even if the full bio-chemical control is not achieved. Therapy with pasireotide also results in tumor volume shrinkage, being beneficial for patients with functioning pituitary adenomas awaiting surgery. Furthermore, due to the high rate of hyperglycemia, pasireotide might be considered as a second-line therapy in patients with metastatic NETs, who do not respond to the old SSAs. Due to the significant risk of hyperglycemia, all pasireotide-treated patients need careful monitoring of fasting and postprandial glucose levels. [2]
2. pasireotide, has recently been approved for the treatment of acromegaly. Areas covered: 1) pasireotide’s pharmacokinetics and pharmacodynamics; 2) pasireotide’s anti-secretory and anti-proliferative effects, from preclinical studies up to phase III clinical trials; and 3) the adverse effects of pasireotide, focusing on hyperglycemia; 4) biomarkers of response to SA treatment.[3]
Systhesis
In the prior art, the mainstream synthesis method of pareptide is solid-phase synthesis method. For example, according to the classical Fmoc / TBU strategy, cyclic [(2R) - 2-phenylglycyl-d-tryptophan-l - (n-tert butoxycarbonyl) lysine-o-benzyl-l-tyrosyl-l-phenylalanyl - (4R) - 4 - [(2-aminoethyl) carbamoyloxy] - l-prolyl] peptide is obtained by solid-phase condensation with sasrinresin or Wang resin. However, there are several common problems in the solid-phase method, such as the need for special equipment, low purity of crude peptides, special purification equipment and high cost. In contrast, the biggest advantage of liquid phase synthesis is the high purity of the target product, which mainly depends on the purification of intermediates. However, how to reasonably design the synthetic route to obtain high yield and how to avoid the high degree of racemization during peptide cyclization remain to be solved.
Figure the systhesis route of Pasireotide
History
Pareptide is an "orphan drug" developed by Novartis pharmaag of Switzerland. It was first approved by the EU drug regulatory authority on April 25, 2012 in accordance with the relevant regulations on rare diseases. Its trade name is signifor. It was approved by the US FDA on December 14, 2012, It is used to treat adrenocortical hyperthyroidism and Cushing's disease that cannot be treated by surgery. After that, it has been approved to be listed in 48 countries.
Toxicity
Pasireotide is a synthetic long-acting cyclic peptide with somatostatin-like activity. Pasireotide activates a broad spectrum of somatostatin receptors, exhibiting a much higher binding affinity for somatostatin receptors 1, 3, and 5 than octreotide in vitro, as well as a comparable binding affinity for somatostatin receptor 2. This agent is more potent than somatostatin in inhibiting the release of human growth hormone (HGH), glucagon, and insulin. Pasireotide is a six-membered homodetic cyclic peptide composed from L-phenylglycyl, D-tryptophyl, L-lysyl, O-benzyl-L-tyrosyl, L-phenylalanyl and modified L-hydroxyproline residues joined in sequence.
Reference
1., "Pasireotide for the treatment of acromegaly,".
2.Sawicka-Gutaj N., Owecki M. & Ruchala M., "Pasireotide - Mechanism of Action and Clinical Applications," Curr Drug Metab, Vol.19, No.10(2018), pp.876-882.
3.Vitale G., Dicitore A. & Sciammarella C. et al., "Pasireotide in the treatment of neuroendocrine tumors: a review of the literature," Endocr Relat Cancer, Vol.25, No.6(2018), pp.R351-R364.
You may like
Related articles And Qustion
See also
Lastest Price from Pasireotide manufacturers
US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00/g2025-04-21
- CAS:
- 396091-73-9
- Min. Order:
- 1g
- Purity:
- 98%min
- Supply Ability:
- 1000g