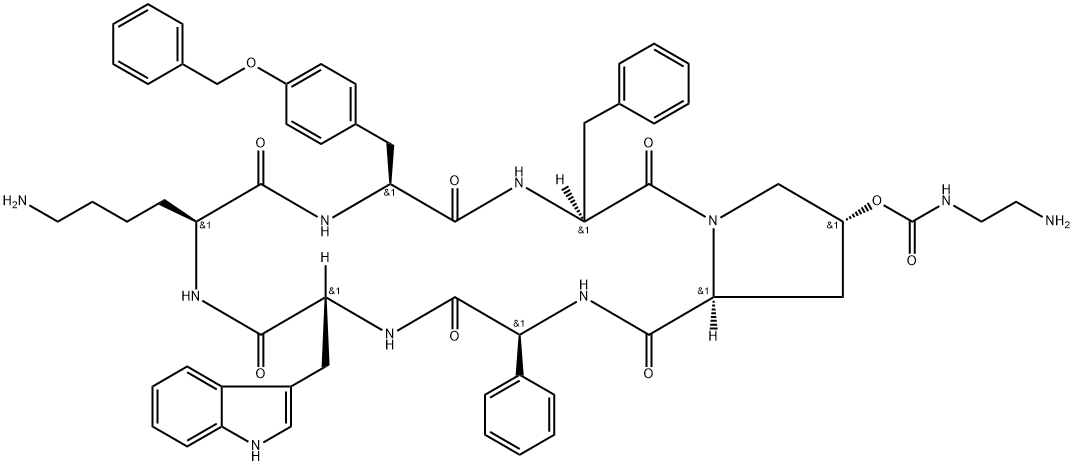
Pasireotide for Cushing's disease
Pasireotide is a man-made protein that is similar to a hormone in the body called somatostatin. Pasireotide is used to treat Cushing's disease or acromegaly (endocrine disorders). Pasireotide is usually given after surgery or other treatments did not work or have stopped working.
It was developed by Novartis. Pasireotide is a somatostatin analog with a 40-fold increased affinity to somatostatin receptor 5 compared to other somatostatin analogs. Pasireotide LAR (the long-acting-release formulation) was approved by the FDA for treatment of acromegaly in December 2014, and had been approved for this indication by the EMA in September 2014.
Cushing's disease Treatment
Pasireotide is a somatostatin receptor ligand approved by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) for treatment of Cushing’s disease. Somatostatin is mainly a somatotropin release-inhibiting factor. At the level of the anterior pituitary, somatostatin inhibits secretion of ACTH in addition to growth hormone (GH), prolactin, and thyrotropin. Peripherally, it acts at the level of the gastrointestinal tract to slow gastric motility, and at the pancreas to inhibit release of insulin, glucagon, and pancreatic polypeptide.
Mechanism of Action
There are five somatostatin receptor subtypes (SSTR1–SSTR5). Each subtype is expressed in varying amounts throughout the body, and they are variably expressed in different tumors. The SSTR5 is the predominant receptor type expressed by corticotrope adenomas, while the predominant receptor type expressed by GH-producing adenomas is SSTR2.
Somatostatin has a very short half-life (approximately 3 min), thus precluding its use as a long-term suppressive therapy. Over the last several decades, somatostatin analogs with longer half-lives have been developed. Octreotide and lanreotide predominantly bind to SSTR2 and have been used successfully in the treatment of acromegaly and secretory neuroendocrine tumors of the gut and pancreas, but they rarely have been effective in Cushing’s disease. Pasireotide exhibits high affinity binding to SSTR5 as well as SSTR1, SSTR2, and SSTR3. Pasireotide has a 40-fold greater affinity for SSTR5 compared to other somatostatin receptor ligands, and correspondingly demonstrates greater efficacy in the treatment of Cushing’s disease].
Acromegaly Treatment
Pasireotide (SOM230), a novel somatostatin analogue with affinity for multiple somatostatin receptor subtypes is a promising emerging therapy for acromegaly. Initial studies have shown this drug to be as effective as the currently available somatostatin analogues. The effect on IGF-1 levels by pasireotide appears to be more sustained compared with octreotide, suggesting a longer half-life. Further long-term studies examining the effect on tumor shrinkage and sustained biochemical response are presently under way to determine whether pasireotide is a reasonable chronic treatment option in acromegaly.
Mechanism of Action
Somatostatin analogs, the mainstay of medical therapy for patients with acromegaly, have been traditionally used after surgery but more recently as first-line therapy in selected patients. Control of GH or IGF-I with adjuvant somatostatin analog therapy is achieved in 48–67% of patients. In recent studies of first-line therapy with octreotide long-acting release (LAR) in patients with de novo acromegaly, combined control of GH and IGF-I after 24 wk of treatment was achieved by approximately 25% of patients. Approximately 90% of GH-secreting pituitary tumors express somatostatin receptor subtypes 2 and 5 (sst2 and sst5), which signal the pituitary gland to suppress GH secretion. Octreotide and lanreotide both act preferentially via sst2 and have lower affinity for sst5.
Side Effects
The most common side effects include hyperglycaemia (high blood sugar levels), diabetes, diarrhoea, abdominal pain (stomach ache), nausea (feeling sick), cholelithiasis (gallstones), injection site reactions, and tiredness.
Additional adverse effects of pasireotide are similar to other somatostatin analogs, and predominantly include gastrointestinal side effects, such as nausea, vomiting, diarrhea, biliary sludge, gallstones, transient elevations of liver function tests (LFTs), and the risk of QT prolongation on electrocardiogram (ECG). With these adverse effects in mind, baseline assessment includes: LFTs, thyroid function tests, insulin-like growth factor 1 (IGF-1) levels, fasting plasma glucose levels, HbA1c, right upper quadrant ultrasound, and an ECG. Finger-stick blood glucose should be measured frequently during the first week of therapy in all patients and then tailored based on the degree of hyperglycemia and the need for treatment. Additional parameters listed should then be reassessed at 3–4-month intervals based on the patients’ symptoms or dose adjustments.
Although the large phase 3 trial showed that patients with mild UFC elevation responded better, there have been other attempts to predict the response to pasireotide.
You may like
Related articles And Qustion
Lastest Price from Pasireotide manufacturers
US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00/g2025-04-21
- CAS:
- 396091-73-9
- Min. Order:
- 1g
- Purity:
- 98%min
- Supply Ability:
- 1000g