Efficacy and safety of sugammadex sodium in reversing rocuronium-induced neuromuscular blockade in children: An updated
ABSTRACT
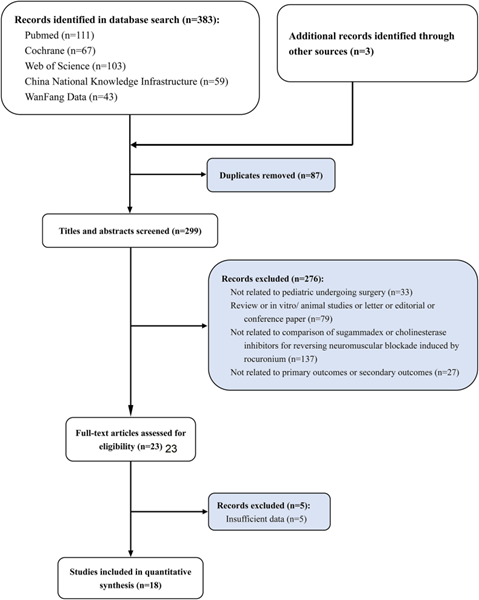
Objective: In response to the differences in pharmacodynamic and pharmacokinetic characteristics of neuromuscular blocking agents between children and adults and limited studies which existing meta-analyses included, this study will update the safety and efficacy of sugammadex (Sug) sodium in reversing rocuronium-induced neuromuscular blockade in children. Methods: Five electronic databases were searched for clinical trials on the safety and efficacy of Sug sodium in reversing rocuronium-induced neuromuscular block in children. A random-effects model was used to calculate the standardized mean difference (SMD) for primary outcomes. The relative risk (RR) was calculated for secondary outcomes. Results: As of 2022-11-03, 18 out of 236 studies included 724 children in the intervention group and 478 children in the control group for meta-analysis. The results showed that compared with the control group, the time required for Train-of-Four Ratio (TOFR) to return to 0.9 and the extubation time were shortened in both 2 mg/kg and 4 mg/kg of Sug sodium, with statistically significant differences (TOFR ≥0.9: 2 mg/kg: SMD = − 2.90; 95%CI: − 3.75, − 2.04; 4 mg/kg: − 3.31; − 4.79, − 1.84; extubation time: 2 mg/kg: − 2.95; − 4.04, − 1.85; 4 mg/kg: − 1.57; − 1.90, − 1.23). Compared with the control group, the total incidence of adverse effects in the Sug group was lower (RR = 0.44; 0.24,0.82). Conclusions: This review and meta-analysis suggest that Sug sodium is more effective and safer in reversing rocuronium-induced neuromuscular blockade in children than traditional antagonistic regimens or placebos.
Introduction of Sugammadex sodium
Sugammadex sodium is an organic sodium salt that is the octasodium salt of sugammadex. Used for reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. It has a role as a neuromuscular agent. Sug sodium is the world’s first and only approved specific binding neuromuscular blockade antagonist. It is a particular aminosteroid antagonist neuromuscular relaxant, a chemically modified cyclodextrin with a three-dimensional structure similar to a hollow cone. The outer structure contains a hydroxyl polar group, which is hydrophilic, and there is a hydrophobic cavity inside.
The drug is captured into the cyclodextrin through hydrophobic interaction to form a water-soluble chelate. A compact chelate is formed at a 1: 1, reaching equilibrium with a very high binding rate and a meager dissociation rate to create a stable chelate. After entering the bloodstream, Sug sodium combines with neuromuscular relaxants to produce an antagonistic muscle relaxant effect. The principle is that Sug sodium is immediately distributed in the extracellular fluid to encapsulate rocuronium or vecuronium bromide molecules, reducing muscle relaxants' concentration in the tissue around the neuromuscular junction, thereby creating a concentration difference with the neuromuscular junction. The muscle relaxant molecules are transferred to the surrounding tissue and encapsulated by Sug sodium, reducing their plasma concentration and creating a gradient between the plasma and the neuromuscular junction.
Discussion
The primary outcome indicators of this study showed that Sug sodium resulted in faster recovery from the neuromuscular blockade in pediatric patients. The time required to reach TOFR ≥0.9 and extubation is shortened, which means that patients recover faster. It improves the efficiency of anesthesiologists and facilitates quick turnaround in the operating room. However, there are still some points to note for this result. First, all the included studies used TOFR ≥0.9 as the recovery standard of neuromuscular block, but many kinds of neuromuscular block monitoring are used in clinical work. For example, an EMG-type neuromuscular block monitor evaluates the depth of neuromuscular block by detecting muscle compound action potential.
In contrast, an MMG-type neuromuscular block monitor evaluates the depth of neuromuscular block by directly or indirectly detecting muscle contraction force. Different types of monitors differ in working mechanisms, and their results are also susceptible to various factors . Second, the results of this study only suggested that Sug sodium could reverse the effect of moderate neuromuscular blockade (T2 or T3 reappearance) or profound neuromuscular blockade (PTC<2 or 2–3) in pediatric patients and could not explain whether Sug sodium was equally effective in mild neuromuscular blockade or intense neuromuscular blockade. Third, Sug sodium can also be used for emergency treatment of patients who are "unable to intubate, unable to ventilate" or "emergency treatment of allergy to rocuronium bromide". Such studies were not included in this meta-analysis.
Secondary outcomes of this study showed that pediatric patients tolerated Sug well. Compared with the control group using cholinesterase inhibitors, sodium gluconate did not increase the incidence of adverse effects, especially more serious ones. The incidence of tachycardia in the Sug sodium group was lower than that in the control group, which may be related to using a larger dose of atropine in the control group. As mentioned above, anticholinergic drugs used to eliminate the adverse effects of cholinesterase inhibitors may bring other negative consequences.
In this study, we did not find a statistically significant difference in the incidence of bradycardia between the Sug sodium group and the control group. However, a recent paper by Arends J et al. on 99 children using Sug sodium showed that bradycardia was lower in children with congenital heart disease. Even if it occurred, there was no need to deal with it. Therefore, compared with the dangerous combination of cholinesterase inhibitors and anticholinergic drugs, Sug sodium alone could stabilize hemodynamics. In addition, it was worth noting that the incidence of nausea and vomiting in the Sug sodium group was lower than in the control group. It was consistent with the conclusion of Vargas M et al. in an adult study. However, no matter in the Sug sodium group or the control group, the incidence of nausea and vomiting was higher than that reported in the adult study, and this phenomenon might be related to the characteristics of anesthesia in children.
References:
[1] SHENG ZHOU; Jianfen R; Haiying Hu. Efficacy and safety of sugammadex sodium in reversing rocuronium-induced neuromuscular blockade in children: An updated systematic review and meta-analysis.[J]. Heliyon, 2023. DOI:10.1016/j.heliyon.2023.e18356.
You may like
See also
Lastest Price from Sugammadex sodium manufacturers

US $1.00/kg2025-07-08
- CAS:
- 343306-79-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000

US $0.00-0.00/kg2025-07-01
- CAS:
- 343306-79-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1T