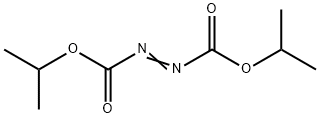
Diisopropyl azodicarboxylate: self-accelerating decomposition and explosion
Description
The Mitsunobu reaction is considered an essential reaction because of its great applications in organic synthesis. It was discovered by Oyo Mitsunobu during the mid-twentieth century. A dehydrative redox reaction converts an alcohol to an ester by coupling it with an acid/pronucleophile. The pronucleophiles include oxygen pronucleophiles, such as carboxylic acids; nitrogen pronucleophiles, such as imides and sulfonamides; and sulfur pronucleophiles, such as thiols, etc. This reaction is mediated by phosphines, i.e., triphenylphosphine, and azodicarboxylates, i.e., diethyl azodicarboxylate (DEAD), diisopropyl azodicarboxylate (DIAD), etc., and a solvent[1].
As an important intermediate in organic synthesis, DIAD is widely used in polymer material synthesis. DIAD is typically used for Mitsunobu reactions. Similar reagents to DIAD include DEAD, DtBAD, and ADDP. However, DIAD is quickly replacing DEAD as the preferred azodicarboxylate due to DEAD's known safety concerns.
Synthesis method
The synthesis method of diisopropyl azodicarboxylate comprises the following steps: (1) adding alkali and a solvent under the protection of inert gases, sequentially adding carbazic acid isopropyl ester and diethyl carbonate, carrying out the heating reaction at 100-180 DEGC for 2-6 hours, adjusting the pH value of a solution to 4.5-7.5 with acid, and carrying out suction filtration, to obtain hydrogenated diisopropyl azodiformate; and (2) adding 40%-50% of sulfuric acid solution to the hydrogenated diisopropyl azodiformate at -20 DEG C to 25 DEG C, dissolving, adding a catalyst, controlling the reaction temperature to -15 DEG C to -5 DEG C, dropwise adding 10%-15% of hydrogen peroxide until no heat is emitted, further reacting for 5-10 hours after dropwise adding is ended, quenching, extracting, washing to be neutral, drying, filtering, and concentrating on obtaining the diisopropyl azodiformate. This synthesis method disclosed by the invention is clean and environmentally friendly in raw material, mild in reaction conditions, and few in byproducts; and the product is high in yield and beneficial to industrialized production.
Uses
DIAD, an essential member of such cyclization reagent, diisopropyl azodiformate cannot only be used for the synthesis of products such as photosensitizers, polymerizing catalysts, and sterilant, also can be used as a liquid blowing agent but itself and plastic cement compatibility good, degradation production is colorless, pollution-free, odorless, has an extensive future[2].
Safety
DIAD belongs to the azo compound, and it can be used to initiate the polymerization reaction between the polymer monomers because it‘s easy to decompose and form free radicals; meanwhile, because of this performance and exothermic reaction characteristics of DIAD, this substance is elementary to decompose and release heat at a low temperature, resulting in self-accelerating decomposition, and finally leading to thermal runaway situation of the reaction. Recently, according to UN-GHS (Globally Harmonized System of Classification and Labelling of Chemicals), DIAD belongs to the typical self-reactive substance, classified as the 8th item, which exists a risk of loss of control due to self-heating reaction during storage or transportation. Once, during production, transportation, application, and storage, when shocked, rubbed, impacted, or influenced by other external stimulation, DIAD can trigger self-accelerating decomposition, resulting in fire or explosion accidents and finally causing severe casualties and property losses[3].

Hence, Jia et al. Thermal decomposition behavior and decomposition mechanism of DIAD were characterized and analyzed by C80 micro-calorimetery and STA-FTIR-MS system. The thermal hazard of DIAD was assessed by using self-accelerating decomposition temperature (SADT) as the evaluation index. The results show that only one exothermic peak appeared in the decomposition process and that the onset temperature and peak temperature increased with the heating rate, whereas the released reaction heat at the heating rates remained approximately constant at 945.67±23.45 kJ/kg. Based on C80 data, the calculated activation energy of DIAD decomposition ranges from 45 kJ/mol to 77 kJ/mol by the Friedman method, while the reaction progresses in the range of 0.10–0.90. During the thermal decomposition process of DIAD, there is only one stage of weight loss, which starts at about 80 ℃, reaches the maximum rate of weight loss at 138 ℃, and ends at about 150 ℃. The thermal decomposition mechanism of DIAD can be mainly summarized as the route that the C-O single bond breaks at first, then C-C breaks, and the N=N bond breaks subsequently. The C-O bond or the C-N bond breaks, final products such as H2O, CO2 etc. appear from further dissociation and oxidation of molecular ionic fragments. According to the above-mentioned results, the calculated SADT of DIAD is 89.0 ℃ based on the Semenov model when packed in a 25kg standard package.
References
[1] Saba Munawar. “Mitsunobu Reaction: A Powerful Tool for the Synthesis of Natural Products: A Review.” Molecules (2022).
[2] CN104478758A - Synthesis method of diisopropyl azodicarboxylate - Google Patents
[3] Min Jia . “Thermal decomposition mechanism of diisopropyl azodicarboxylate and its thermal hazard assessment.” Thermochimica Acta 688 (2020): Article 178601.
You may like
Related articles And Qustion
See also
Lastest Price from Diisopropyl azodicarboxylate manufacturers
US $0.00/kg2025-11-21
- CAS:
- 2446-83-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs
US $0.00/kg2025-10-31
- CAS:
- 2446-83-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise