Dabigatran Etexilate: A Potent Thrombin Inhibitor for Stroke Prevention in Non-Valvular Atrial Fibrillation
General Description
Dabigatran Etexilate is a prodrug that converts into dabigatran, a potent inhibitor of thrombin, which selectively inhibits the formation of blood clots. It has a rapid pharmacokinetic profile, with linear dose-dependent increases in Cmax and AUC. It has moderate tissue distribution and is predominantly eliminated via renal excretion. The therapeutic efficacy of Dabigatran Etexilate was assessed in the RE-LY trial, demonstrating noninferiority to warfarin in preventing stroke or systemic embolism in patients with NVAF and a risk of stroke. The 150 mg dosage was superior to warfarin in reducing certain events, while the 110 mg dosage showed noninferiority or lower rates of haemorrhagic stroke and hospitalization. Individual patient factors should be considered when selecting the appropriate dosage.

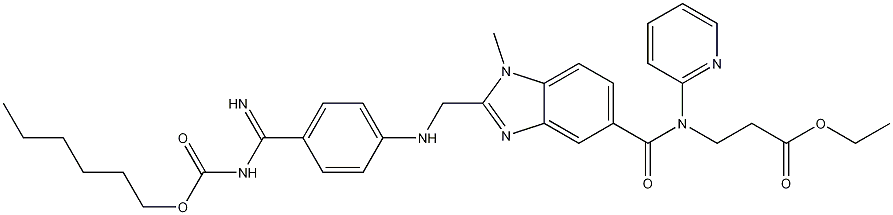
Figure 1. Dabigatran etexilate
Pharmacodynamic Profile
Dabigatran Etexilate is a prodrug that converts into dabigatran, a potent inhibitor of thrombin. It selectively inhibits thrombin, preventing the formation of blood clots. Laboratory studies have shown its high selectivity for thrombin over other serine proteases in the coagulation cascade. It effectively inhibits thrombin-induced platelet aggregation and tissue factor-induced thrombin generation. Oral administration of Dabigatran Etexilate dose-dependently prolongs various blood coagulation parameters, with maximum effects occurring within 2 hours of administration. Routine anticoagulation monitoring is not necessary, but measuring dabigatran-related anticoagulation may be helpful in identifying individuals at risk of excessive bleeding. Biomarker substudies have shown that Dabigatran Etexilate significantly reduces D-dimer and apolipoprotein B levels in patients with non-valvular atrial fibrillation compared to warfarin. Elevated D-dimer levels are associated with increased stroke or major bleeding risk. Growth differentiation factor 15 is identified as a risk marker for all-cause mortality and major bleeding in these patients. Further research is needed to understand the impact of Dabigatran Etexilate on apolipoprotein B metabolism. 1
Pharmacokinetic Profile
Dabigatran Etexilate is a medication used for the prevention of stroke or systemic embolism in patients with non-valvular atrial fibrillation. Its pharmacokinetic profile shows that the drug is rapidly absorbed after oral administration and is completely converted to dabigatran via esterase-catalyzed hydrolysis in plasma and in the liver. In healthy volunteers receiving oral dabigatran etexilate, Cmax values were reached within 2 hours, and the drug exhibited linear pharmacokinetics, with AUC and Cmax values increasing in a dose-dependent manner. Dabigatran has an absolute oral bioavailability of around 6.5%. The volume of distribution of dabigatran is 60-70 L, indicating moderate tissue distribution, and plasma protein binding of dabigatran is relatively low at 35%. Elimination of dabigatran occurs predominantly via renal excretion of unchanged drug, with the renal clearance corresponding to the glomerular filtration rate. The terminal half-life of dabigatran is around 12-14 hours after repeated administration. Overall, understanding its pharmacokinetic profile can help clinicians optimize dosing and minimize adverse effects of Dabigatran Etexilate. 2
Therapeutic Efficacy
The therapeutic efficacy of Dabigatran Etexilate, a medication used for the prevention of stroke or systemic embolism in patients with non-valvular atrial fibrillation (NVAF), was assessed in the RE-LY trial. This large, phase III, multicenter trial compared the efficacy of Dabigatran Etexilate with warfarin. The study included patients aged 18 years or older with documented NVAF and at least one risk factor for stroke. The baseline demographics and clinical characteristics were similar between the treatment groups. The patients were randomized to receive either Dabigatran Etexilate 110 mg or 150 mg twice daily, or open-label warfarin. The results showed that both dosages of Dabigatran Etexilate were noninferior to warfarin in preventing stroke or systemic embolism. However, the 150 mg dosage was superior to warfarin, while the 110 mg dosage was not significantly different from warfarin in terms of preventing stroke or systemic embolism. Dabigatran Etexilate 150 mg twice daily was associated with lower rates of certain events, including any stroke, disabling/fatal stroke, and death from vascular causes, compared to warfarin. The 110 mg dosage showed lower rates of haemorrhagic stroke and hospitalization compared to warfarin. Overall, Dabigatran Etexilate demonstrated therapeutic efficacy in preventing stroke and systemic embolism in patients with NVAF and a risk of stroke. The 150 mg dosage showed superiority over warfarin, while the 110 mg dosage showed noninferiority. The choice of dosage should be based on individual patient factors and considerations. 3
Reference
1. Blair HA, Keating GM. Dabigatran Etexilate: A Review in Nonvalvular Atrial Fibrillation. Drugs. 2017;77(3):331-344.
2. Sanford M, Plosker GL. Dabigatran etexilate. Drugs. 2008;68(12):1699-1709.
3. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
Related articles And Qustion
See also
Lastest Price from Dabigatran etexilate manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 211915-06-9
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $500.00-300.00/kg2025-04-21
- CAS:
- 211915-06-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000