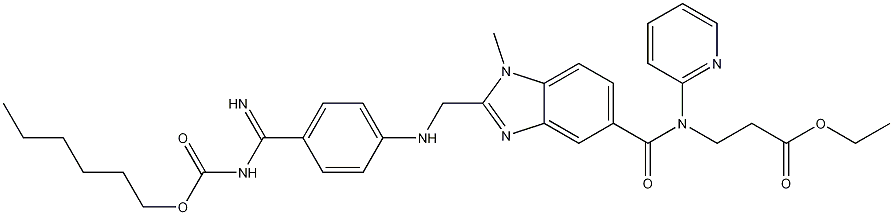
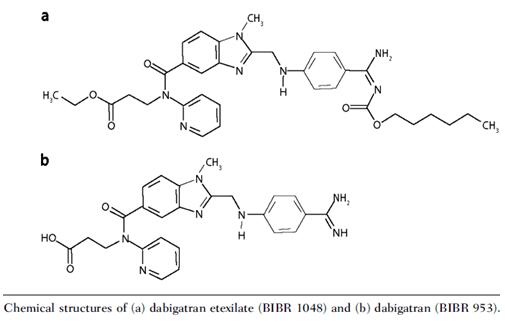
Dabigatran etexilate: clinical applications and safety
General Description
Dabigatran etexilate is commonly used for the clinical treatment of acute venous thromboembolism (VTE) and secondary prevention of VTE in pediatric patients. In the DIVERSITY trial, it showed comparable efficacy to standard of care treatments, achieving the primary endpoint of complete thrombus resolution, freedom from recurrent VTE, and freedom from VTE-related death in 46% of dabigatran etexilate recipients and 42% of standard of care recipients after 3 months of treatment. For secondary prevention of VTE, dabigatran etexilate demonstrated effectiveness and safety in a phase 3 safety study, with a low recurrence rate and minimal development of post-thrombotic syndrome. Regarding its safety profile, most adverse events were mild or moderate, with bleeding being the most common serious adverse event, occurring in a small percentage of patients. Dose adjustments were occasionally required due to elevated plasma dabigatran concentrations. Discontinuation of treatment and deaths related to the use of dabigatran etexilate were rare. In summary, dabigatran etexilate offers favorable clinical outcomes and a manageable safety profile for treating and preventing VTE in pediatric patients.

Figure 1. Capsules of dabigatran etexilate
Clinical applications
Acute Venous Thromboembolism
Dabigatran etexilate was evaluated for its clinical application in the treatment of acute venous thromboembolism in pediatric patients in the DIVERSITY trial. This multinational, randomized, open-label phase 2b/3 trial aimed to compare the efficacy of dabigatran etexilate with standard of care (SOC) treatments such as LMWH, UFH, VKA, and fondaparinux over a 3-month period. The trial enrolled patients below 18 years of age with diagnoses of deep vein thrombosis, pulmonary embolism, central line thrombosis, or sinus vein thrombosis. Patients were required to have received LMWH or UFH treatment and anticipated needing anticoagulation therapy for at least 3 months. Exclusion criteria included certain age and weight restrictions, increased bleeding risk, kidney dysfunction, hepatic disease, infective endocarditis, and heart valve prosthesis requiring anticoagulation. Dabigatran etexilate and SOC were compared using a 2:1 randomization, stratified by age groups. Dabigatran etexilate was administered as capsules, pellets, or oral solution based on age and weight. The majority of dabigatran etexilate recipients received capsules. The SOC group primarily received VKA or LMWH therapy. The primary composite efficacy endpoint measured complete thrombus resolution, freedom from recurrent VTE, and freedom from VTE-related death. Dabigatran etexilate demonstrated non-inferiority to SOC in achieving this endpoint. After 3 months of treatment, 46% of dabigatran etexilate recipients and 42% of SOC recipients achieved the primary endpoint. The individual components of the endpoint, including freedom from recurring VTE and VTE-related death, were comparable between the two groups. Partial thrombus resolution was seen in 32% of dabigatran etexilate recipients and 28% of SOC recipients, while complete or partial thrombus resolution was observed in 78% and 70% of patients, respectively. In conclusion, the DIVERSITY trial demonstrated that dabigatran etexilate is an effective treatment option for acute VTE in pediatric patients, providing comparable clinical outcomes to SOC treatments. 1
Secondary Prevention of Venous Thromboembolism
Dabigatran etexilate has been evaluated for its use in secondary prevention of venous thromboembolism in pediatric patients aged under 18 years. The study was an open-label, single-arm, phase 3 safety study involving 203 participants stratified by age and treated with dabigatran etexilate for up to 12 months. The primary endpoints of the study were recurrence of VTE, overall and thrombotic/thromboembolic event mortality, major and minor bleeding events, and clinically relevant non-major bleeding events. The recurrence of VTE was low with dabigatran etexilate treatment, with only two patients experiencing recurrent VTE within 3 to 6 months of treatment. The risk factors for these cases included methylenetetrahydrofolate reductase homozygous mutation, protein S deficiency, and plasminogen activator inhibitor-1 heterozygote mutation. The likelihood of having freedom from recurrent VTE was high across all age groups after treatment. Patients who experienced deep vein thrombosis or central-line thrombosis as their most recent VTE and were treated with dabigatran etexilate had a low likelihood of developing post-thrombotic syndrome after treatment and follow-up. Overall, dabigatran etexilate appears to be effective and safe for use in secondary prevention of VTE in pediatric patients. 2
Safety
Dabigatran etexilate is generally well tolerated in pediatric patients for the treatment of acute venous thromboembolism (VTE) and for secondary prevention of VTE. In clinical trials, high acceptability was reported for all formulations of dabigatran etexilate used. The most common adverse events (AEs) experienced by patients were mostly mild or moderate in severity. The incidence of AEs with dabigatran etexilate was similar across different age groups, while the standard of care (SOC) group had a higher proportion of AEs in patients aged 12 to <18 years compared to those aged <2 years. Serious AEs (SAEs) occurred in a small percentage of patients receiving dabigatran etexilate, with bleeding being the most common SAE. Vascular disorders and gastrointestinal disorders were also reported as SAEs. More patients aged 12 to <18 years experienced SAEs compared to those aged <2 years in both the dabigatran etexilate and SOC groups. Dose adjustments were required for a portion of patients, with some needing adjustments due to high plasma dabigatran concentrations. AEs resulting in treatment discontinuation were relatively low, and no deaths were reported specifically related to dabigatran etexilate use. Long-term safety data showed that dabigatran etexilate had a similar tolerability profile when used for up to 12 months in the secondary prevention of VTE. Recurrent VTE and bleeding events were reported in a small percentage of patients, with major bleeding events being rare. Epistaxis, upper abdominal pain, dyspepsia, pyrexia, nasopharyngitis, vomiting, and headache were the most common AEs reported during treatment. Overall, the findings suggest that dabigatran etexilate is well tolerated with a manageable safety profile in pediatric patients for the treatment and prevention of venous thromboembolism. 3
Reference
1. Halton J, Brandão LR, Luciani M, et al Dabigatran etexilate for the treatment of acute venous thromboembolism in children (DIVERSITY): a randomised, controlled, open-label, phase 2b/3, non-inferiority trial. Lancet Haematol. 2021;8(1):e22–33.
2. Brandão LR, Albisetti M, Halton J, et al Safety of dabigatran etexilate for the secondary prevention of venous thromboembolism in children. Blood. 2020;135(7):491–504.
3. Brandão L, Tartakovsky I, Halton J, et al Efficacy and safety of dabigatran in the treatment and secondary prevention of venous thromboembolism in children with cerebral venous and sinus thrombosis [abstract no. PB0787]. In: ISTH 2021 Congress. 2021.
Related articles And Qustion
See also
Lastest Price from Dabigatran etexilate manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 211915-06-9
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $500.00-300.00/kg2025-04-21
- CAS:
- 211915-06-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000