Butyl isocyanate: activities, toxicity and applications
General Description
Butyl isocyanate, a breakdown product of the fungicide benomyl, was studied for its impact on yeast metabolism. It was found that butyl isocyanate inhibited yeast respiration more strongly than carbendazim, another breakdown product of benomyl, while its inhibition of fermentation was lower. Hexyl isocyanate exhibited the highest inhibition of both respiration and fermentation. Toxicity studies conducted on Xenopus laevis embryos revealed that butyl isocyanate caused severe external malformations and tissue-specific abnormalities. Comparatively, carbendazim showed stronger inhibition of neural tissue induction than butyl isocyanate. In terms of applications, butyl isocyanate is important for synthesizing benzimidazole fungicides used for fungal disease control. It also plays a role in the production of sulfonylurea derivatives, which are used in diabetes treatment. Additionally, butyl isocyanate acts as a catalyst in the synthesis of sulfonylurea herbicides for weed control in agriculture. Overall, butyl isocyanate has diverse applications in pharmaceuticals, pesticides, and dyestuffs, contributing to advancements in medicine, agriculture, and the chemical industry.

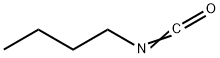
Figure 1. Butyl isocyanate
Activities
The activity of butyl isocyanate was examined in relation to yeast metabolism. The researchers measured yeast respiration and fermentation to assess the impact of fungicidal chemicals on these processes. It was found that benomyl, a fungicide, inhibited both respiration and fermentation more than its breakdown product, carbendazim. However, butyl isocyanate, another breakdown product of benomyl, exhibited a higher inhibition of respiration but a lower inhibition of fermentation compared to the parent compound. Among the tested isocyanates, hexyl isocyanate was the most inhibitory toward both activities. Captan was found to be more active, while iprodione was less active than benomyl. Furthermore, when benomyl was prepared in 80% ethanol, it rapidly broke down to carbendazim, resulting in only 59% of the dissolved benomyl remaining intact during experimentation with yeast. Overall, this research sheds light on the activity of butyl isocyanate and other fungicidal compounds on yeast metabolism, highlighting their differing effects on respiration and fermentation processes. 1
Toxicity
Butyl isocyanate is a toxic compound that was investigated for its effects on development in Xenopus laevis, the African clawed frog. In the study, frog embryo teratogenesis assays were performed to test the toxic effects of BIC. Exposure to concentrations of BIC ranging from 0 to 0.2 microM resulted in severe external malformations in the embryos. Histological examinations showed dysplasia of the brain, eyes, intestine, and somatic muscle, as well as swelling of the pronephric ducts. The addition of Butyl isocyanate weakly inhibited the induction of neural tissues, while carbendazim, another compound tested in the study, strongly inhibited neural tissue induction. Electron micrographs revealed degeneration of cell junctions in the carbendazim-treated group, but not in the Butyl isocyanate-treated group. Both test groups showed numerous residual yolk platelets and mitochondrial degeneration. Gene expression analysis showed that the expression of the neural-specific marker neural cell adhesion molecule was more strongly inhibited in the carbendazim-treated group compared to the Butyl isocyanate-treated group. Overall, Butyl isocyanate demonstrated toxic effects on development, leading to severe malformations and tissue-specific abnormalities in the frog embryos. 2
Applications
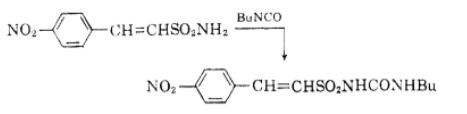
Butyl isocyanate is a versatile compound with various applications in the synthesis of pharmaceuticals, pesticides, and dyestuff products. It serves as an important raw material or intermediate in these industries. In the field of pharmaceuticals, butyl isocyanate is utilized for the synthesis of benzimidazole fungicides, which are used to control fungal diseases. Additionally, it is involved in the production of sulfonylurea derivatives, including glibenclamide, tolbutamide, chlorpropamide, and other hypoglycemic sulfonylurea agents. These compounds have sugar-lowering activity and are used in the treatment of diabetes. Furthermore, butyl isocyanate acts as a catalyst in the synthesis of sulfonylurea herbicides like chlorsulfuron, sulfometuron, metsulfuron-methyl, and chlorimuron-ethyl. These herbicides are widely used for weed control in agriculture. Overall, butyl isocyanate plays a crucial role in the synthesis of various pharmaceuticals, pesticides, and dyestuffs, contributing to advancements in the fields of medicine, agriculture, and chemical industry. 3
Reference
1. Chiba M, Bown AW, Danic D. Inhibition of yeast respiration and fermentation by benomyl, carbendazim, isocyanates, and other fungicidal chemicals. Can J Microbiol. 1987 Feb;33(2):157-161.
2. Yoon CS, Jin JH, Park JH, Yeo CY, Kim SJ, Hwang YG, Hong SJ, Cheong SW. Toxic effects of carbendazim and n-butyl isocyanate, metabolites of the fungicide benomyl, on early development in the African clawed frog, Xenopus laevis. Environ Toxicol. 2008 Feb;23(1):131-144.
3. Kucera SP, Swann JM, Kennedy JR, Schultz TW. The effects of benomyl and its breakdown products carbendazim and butyl isocyanate on the structure and function of tracheal ciliated cells. J Environ Sci Health B. 1995 Nov;30(6):779-799.
Related articles And Qustion
See also
Lastest Price from Butyl isocyanate manufacturers

US $15.00-10.00/KG2021-07-13
- CAS:
- 111-36-4
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton

US $15.00-10.00/KG2021-07-10
- CAS:
- 111-36-4
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton