The application of 2,5-Furandicarboxylic acid
Description
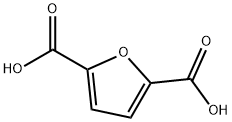
2,5-Furandicarboxylic acid (FDCA), dehydromucic acid, is a furan derivative. FDCA is one of the top-12 value-added chemicals derived from biomass that may serve as a 'green' substitute for terephthalic acid (TPA) in polyesters. On a laboratory scale, it is often synthesized from 5- hydroxymethylfurfural (HMF), which in turn can be obtained from carbohydrate-containing sources such as glucose, fructose, sucrose, and starch. From fructose and glucose, HMF is obtained by acidic eliminating three moles of water[1].

Application
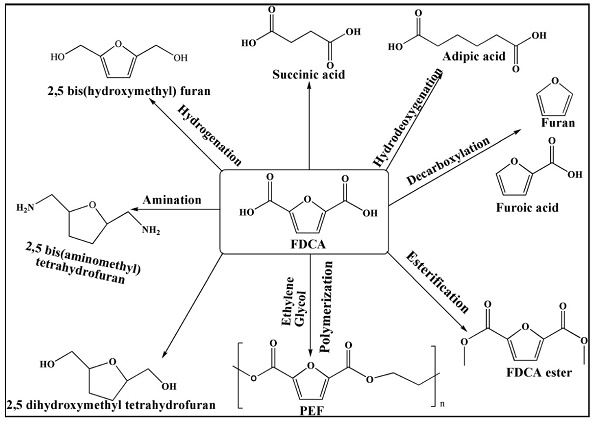
HMF is metabolized into FDCA in human urine and blood plasma in a negligible amount. Mainly FDCA can be used for the production of biochemical like succinic acid, isodecylfuran-2, 5-dicarboxylate, isononyl furan-2, 5-dicarboxylate, dipentyl furan-2, 5-dicarboxylate, diheptyl furan-2, 5-dicarboxylate and poly (ethylene dodecanedioate-2, 5-furandicarboxylate) (PEDF). FDCA is also essential in preparing hexanoic acid, macrocyclic ligands, fungicides, corrosion inhibitors, and thiolene films[2].
FDCA derivatives like 2, 5-dihydroxymethylfuran 2, and 5-bis (hydroxymethyl) tetrahydrofuran could produce new polyesters as alcohol components. It is a highly demanding monomer in the production of dichloride- (FDCDCl), dimethyl- (DMFDC), and bis (hydroxyethyl)- (BHEFDC) derivatives for the production of plasticizers, polyamides, and polyesters. Structurally, furan rings of FDCA are analogous to fossil-derived TPA (Terepthalic Acid), one of the currently used plastic materials widely used today, and FDCA can be an eco-friendly alternative for producing new bioplastics. It can also be used as an alternative to polybutylene terephthalate (PBT) and polyethylene terephthalate (PET) to produce film, fiber, packing materials, and soft drink bottles. As such, FDCA monomer cannot be used in polymer production. This has to be combined with ethylene glycol and synthesize PEF (polyethylene furonate) and PBF (polybutylene furonate). Due to the thermo-chemical, mechanical, gas barrier, and recyclability properties of PEF, this can be used as an alternative to PET and PBT.
There needed to be more production of PEF from industries due to its lower purity, sustainable availability, and the process difficulties of FDCA. FDCA diethyl esters have anesthetic properties similar to cocaine. It has a chelating property with ions (Ca2+, Cu2+, and Pb2+) and is applied as a medicine for kidney stone removal and the synthesis of artificial vein transplantation. Ultimately, this FDCA has been highlighted as one of the 12 platform biochemicals used for in-depth research for sustainable industrial production.
Ecotoxicogenomic study
FDCA, HMF, and TPA are biodegradable in natural soil. However, these chemicals can severely impact the reproduction of F. candida in sterilized soil. Transcriptional changes were examined at EC50 concentrations of FDCA, HMF, and TPA spiked in sterilized LUFA 2.2 soils. The results indicate that FDCA and TPA cause no significant changes in gene expression, which may be due to the low chemical water solubility and, therefore, slow uptake from the pore water. In contrast, many genes were significantly regulated in F. candida after exposure to HMF. Gene ontology analysis showed many biological processes to be significantly affected, such as nucleic acid metabolism, the transcriptional metabolic process, the cell developmental process, and oxidation–reduction process. Moreover, the transcriptional profiles suggest that HMF might be biotransformed by F. candida into 5-sulfoxymethylfurfural (SMF), which is genotoxic and mutagenic. Current research shows that the environmental risk of the FDCA production chain from biomass is relatively low but may only be affected by the release of the intermediate HMF compound.
References
[1] Chen, Guangquan Nico M. van Straalen and Dick Roelofs. “The ecotoxicogenomic assessment of soil toxicity associated with the production chain of 2,5-furandicarboxylic acid (FDCA), a candidate bio-based green chemical building block†.” Green Chemistry 16 (2016): 4420–4431.
[2] Rajendran Omana Rajesh. “Bioengineering advancements, innovations and challenges on green synthesis of 2, 5-furan dicarboxylic acid.” Bioengineered (2020): 19–38.
You may like
Related articles And Qustion
See also
Lastest Price from 2,5-Furandicarboxylic acid manufacturers

US $0.00-0.00/kg2025-12-01
- CAS:
- 3238-40-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 10 tons

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 3238-40-2
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 500kg