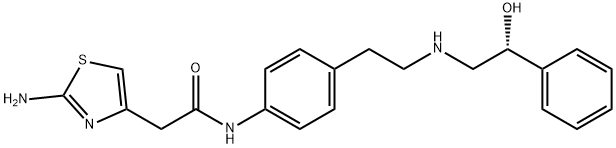
Clinical Research and Mechanism of Action of Mirabegron
Mirabegron is a β3-adrenergic receptor agonist approved in several countries for the symptomatic treatment of adults with overactive bladder syndrome. Mirabegron tablets, developed by the Japanese pharmaceutical company Astellas, were first launched in Japan on September 16, 2011, and received approval from the U.S. Food and Drug Administration (FDA) on June 28, 2012 for the treatment of overactive bladder (OAB) in adults. Mirabegron is an efficacious new treatment for overactive bladder syndrome with a favourable tolerability profile. Comparative trials of mirabegron versus antimu scarinics that are in current use for the treatment of over active bladder syndrome would be of considerable interest.
Figure 1: Pharmaceutical Product Image of Mirabegron
Overview
Through three 12-week, randomized, double-blind, placebo-controlled, multinational trials conducted in patients with overactive bladder syndrome, once-daily oral mirabegron at doses of 25 or 50 mg significantly reduced both the adjusted mean number of incontinence episodes per 24 hours (in patients with incontinence at baseline) and the adjusted mean number of micturition episodes per 24 hours (in the full trial populations), which were defined as coprimary endpoints. Furthermore, across these trials, the 50 mg once-daily dose also consistently and significantly reduced urgency episodes and increased the volume of urine voided per micturition, improvements that were generally associated with enhanced health-related quality of life (HR-QOL) and greater treatment satisfaction. [1]
Clinical Research
Based on descriptive analyses from a 12-month clinical trial, once-daily mirabegron 50 mg and tolterodine extended-release (ER) 4 mg both demonstrated efficacy in reducing urinary symptoms and improving health-related quality of life (HR-QOL). Mirabegron exhibited a generally favorable tolerability profile throughout the studies: over 12 weeks, its adverse event rate was comparable to placebo, and during the 12-month treatment period, only 2.8% of patients receiving mirabegron 50 mg reported dry mouth—significantly lower than the 8.6% incidence observed with tolterodine ER 4 mg. Additionally, mirabegron 50 mg once daily was associated with a low risk of QT interval prolongation. These findings support mirabegron as an efficacious new therapeutic option for overactive bladder syndrome, characterized by a favorable tolerability profile. In a 12-week dose-ranging study, mirabegron demonstrated statistically significant improvements in the primary endpoint of mean number of micturitions per 24 hours, with changes from baseline of -1.9, -2.1, -2.1, and -2.2 for the 25, 50, 100, and 200 mg once-daily dose groups respectively, compared to -1.4 for placebo (p < 0.05 for the 50, 100, and 200 mg doses versus placebo). The drug also showed generally significant superiority over placebo across key secondary urinary endpoints, as evidenced by the 50 mg once-daily dose producing significantly greater reductions in 24-hour incontinence, urgency incontinence, and nocturia episodes (p < 0.05 or p < 0.01) along with a substantial increase in mean voided volume per micturition (p < 0.001), though it did not achieve significant improvements in either 24-hour urgency episodes or the level of urgency per micturition. [1]
Mechanism of Action
Mirabegron is a potent and selective β3-adrenergic receptor agonist that exhibits a distinct mechanism of action in overactive bladder syndrome compared to antimuscarinics, which target parasympathetic nerves. During bladder filling, smooth muscle relaxation relies on sympathetic nerve activity and noradrenaline release. While β1- and β2-adrenergic receptors mediate sympathetic input to the detrusor muscle in most species, β3-adrenergic receptors represent the predominant subtype in the human bladder. Both animal studies and experiments with isolated human bladder tissue have demonstrated that mirabegron induces smooth muscle relaxation. These findings indicate that the therapeutic benefits of mirabegron stem from enhanced detrusor muscle relaxation, leading to increased bladder capacity and reduced micturition frequency. Importantly, as shown in studies of rats with partial urethral obstruction and humans with bladder obstruction, mirabegron does not interfere with bladder emptying—a process primarily regulated by parasympathetic control. [1]
Reference
[1] Mark,Sanford. Mirabegron: A Review of Its Use in Patients with Overactive Bladder Syndrome[J]. Drugs, 2013, 73:1213-1225.
Lastest Price from Mirabegron manufacturers

US $0.00/g2025-04-21
- CAS:
- 223673-61-8
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 1000g

US $25.00/ASSAYS2025-04-21
- CAS:
- 223673-61-8
- Min. Order:
- 100ASSAYS
- Purity:
- 99.5%
- Supply Ability:
- 100 mt