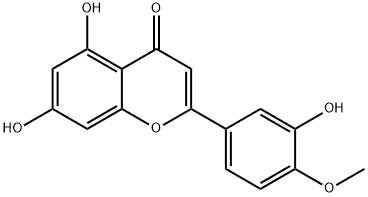
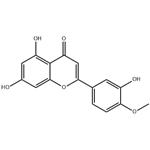
Anticancer Activity and Therapeutic Applications of Diosmetin
Diosmetin, a naturally occurring flavonoid abundantly found in citrus plants such as Citrus sinensis and Rosmarinus officinalis, exhibits distinctive bioactivities attributed to its 3′,5,7-trihydroxy-4′-methoxyflavone structure. Studies have demonstrated that diosmetin exerts antioxidant effects through scavenging reactive oxygen species (ROS) and modulating the Nrf2/HO-1 signaling pathway, while also suppressing the release of pro-inflammatory cytokines including TNF-α and IL-6, thereby confirming its anti-inflammatory potential.

Figure 1: Picture of Diosmetin
Overview
Diosmetin (3',5,7-trihydroxy-4'-methoxyflavone), the aglycone of the flavonoid glycoside diosmin (3',5,7-trihydroxy-4'-methoxyflavone-7-rhamnoglucoside) which occurs naturally in citrus fruits, is also present in the legume Acacia farnesiana Wild and Olea europaea L. leaves. This compound inhibits proliferation of human oral squamous carcinoma SCC-9 cells and acts as a potent inhibitor of CYP1A1 and CYP1B1 enzymes, revealing its protective potential against certain cancers. Pharmacological studies report that diosmetin exhibits anticancer, antimicrobial, antioxidant, estrogenic, and anti-inflammatory activities. Notably, diosmin is hydrolyzed to its aglycone diosmetin by intestinal microflora enzymes prior to systemic absorption. [1]
Anticancer Activity
Studies on U14 cervical cancer-bearing mice demonstrated that diosmetin significantly inhibited tumor growth, modulated serum cytokine levels by elevating interleukin-2 (IL-2) while reducing TNF-α, TGF-β1, and IL-10, and increased the CD4+/CD8+ T lymphocyte ratio compared to controls. In vitro evaluations revealed potent activity against brain carcinoma U251 cells and moderate efficacy against breast carcinoma MCF-7 lines, while metabolic studies showed cytochrome P450 CYP1 enzymes convert diosmetin to luteolin via aromatic demethylation. Although diosmetin inhibited over 70% of 17β-HSD type 1 activity in T-47D estrogen-receptor-positive breast cancer cells, it did not affect their proliferation. Further mechanistic investigations indicated suppression of macrophage M-CSF-induced proliferation via NF-κB pathway inhibition without compromising cellular viability. [1]
Antiinflammatory Activity
Diosmetin demonstrated significant anti-inflammatory effects in the 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse ear edema model, with additional studies confirming its inhibitory activity in TPA-induced inflammation. Further mechanistic studies revealed its capacity to moderately suppress pro-inflammatory cytokine secretion, including IL-6 and TNF-α. In investigating antioxidant properties, diosmetin effectively attenuated bovine serum albumin-derived advanced glycosylation end products (BSA-AGEs)-induced nitric oxide (NO) and TNF-α production, showing dose-dependent inhibition of NO generation and significant reduction in TNF-α expression. Complementary in vitro evaluation using the DPPH model system confirmed its substantial free radical scavenging capacity. Lipopolysaccharides (LPS) released during bacterial infections induce pro-inflammatory cytokine expression and can lead to complications such as neuronal damage and septic shock. In a screening of twenty-nine commercially available polyphenol-containing plant extracts and pure compounds for their capacity to suppress LPS-induced upregulation of nitric oxide (NO) production, diosmetin was identified as an active constituent based on its significant inhibition of NO generation. Complementary in vitro studies using human neuronal cell lines suggest that the increased vascular tone observed following oral administration of diosmetin may be mediated through amine reuptake inhibition at the level of peripheral sympathetic nerves. [1]
Analytical Techniques
Specific, sensitive, and precise HPLC methodologies have been developed and validated for the quantification of diosmetin in human plasma. One validated protocol involves enzymatic hydrolysis of plasma samples with β-glucuronidase/sulfatase, followed by liquid-liquid extraction using tert-butyl methyl ether under acidic conditions (pH 2), with chromatographic separation achieved on a C-18 reversed-phase column using a methanol/1% formic acid (58:42, v/v) mobile phase at a flow rate of 0.5 mL/min. Complementarily, a gas chromatography-mass spectrometry (GC/MS) method has also been established and validated for determining diosmetin levels in both human plasma and urine. Furthermore, an accurate and precise HPLC assay utilizing 7-ethoxycoumarin as an internal standard was developed for reliable plasma diosmetin quantification.
Therapeutic Applications
In neurodegenerative disease models, diosmetin reduces neuronal apoptosis by activating the PI3K/Akt signaling pathway and significantly improves cognitive function. Its anticancer properties involve induction of tumor cell cycle arrest (G2/M phase) and activation of the mitochondrial apoptosis pathway, demonstrating an IC₅₀ of 15.2 μM against breast cancer MCF-7 cells. Regarding vascular protection, diosmetin upregulates eNOS expression and promotes NO production to improve endothelial function. Animal studies indicate an oral bioavailability of only 12.3%, with primary metabolism mediated by the UGT1A1 enzyme to form glucuronide conjugates. Notably, its methylated metabolite demonstrates blood-brain barrier penetration capacity, accounting for the observed central nervous system activity. [1]
Reference
[1] Kanika Patel, A Review on Pharmacological and Analytical Aspects of Diosmetin: A Concise Report. Chin. J. Integr. Med. 2013, 19:792-800.
See also
Lastest Price from Diosmetin manufacturers
US $0.00-0.00/kg2025-09-08
- CAS:
- 520-34-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1

US $10.00/ASSAYS2025-08-20
- CAS:
- 520-34-3
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton