Chemical Reactivity of Tetrahydrofuran
Tetrahydrofuran(THF) is a colorless, volatile, flammable, pleasant-smelling liquid with low viscosity and a bp of 64°C. It is one of the most polar cyclic ethers. THF should not be distilled to dryness because it leaves a residue of high explosive peroxides. The 1 H NMR spectrum in CCl4 showed two peaks at δ 3.61 ppm for C2 –H and C5 –H, while a peak at δ 1.79 ppm is designated for C3 –H and C4 –H protons. The 13C NMR showed two peaks at δ 68.60 ppm for C2 and C5 , while a peak at δ 26.20 ppm was assigned for C3 and C4.

Chemical Reactivity
OXIDATION
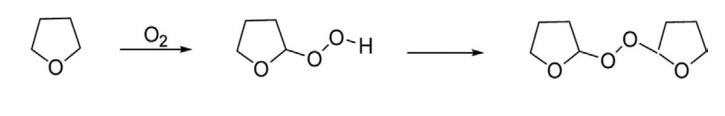
Tetrahydrofuran reacts readily with oxygen to form hydroperoxide and thereafter is transformed to peroxides. The formation of peroxide can be inhibited by addition of certain stabilizers such as 3,5-di-tert-butyl-4-hydroxytoluene.
METALATION
Like furan, THF is also lithiated with n-butyllithium to give 2-lithiotetrahydrofuran,
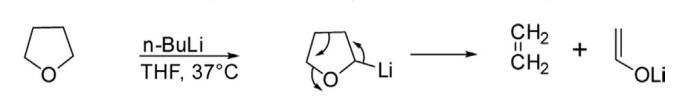
which finally undergoes cycloreversion generating ethylene and acetaldehyde lithium enolate.
Ring-Opening
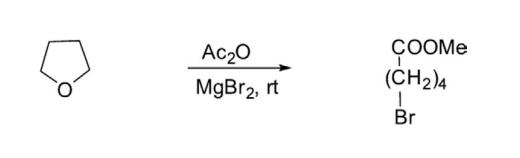
Reaction Ring opening of THF by magnesium bromide and acetic anhydride in acetonitrile has been reported285 to yield methyl 5-bromopentanoate.
You may like
Related articles And Qustion
See also
Lastest Price from Tetrahydrofuran manufacturers

US $0.00/kg2025-06-18
- CAS:
- 109-99-9
- Min. Order:
- 1000kg
- Purity:
- i
- Supply Ability:
- 80MT

US $10.00/KG2025-04-21
- CAS:
- 109-99-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt
![271-89-6 Benzo[b]furanPhysical PropertiesUsesSynthesis](/NewsImg/2024-01-04/6383997625352240343885752.jpg)

![271-89-6 Benzo[b]furanPhysical PropertiesUsesSynthesis](/NewsImg/2022-01-25/6377871824808708721299743.jpg)