A brief description of Tetrahydrofuran
Chemical properties
Tetrahydrofuran, also known as oxacyclopentane, oxolane, tetramethylene oxide, and 1,4-epoxybutane, is a derivative of 1,4-Butanediol. Molecular formula: C4H8O, molecular weight: 72.1057.;
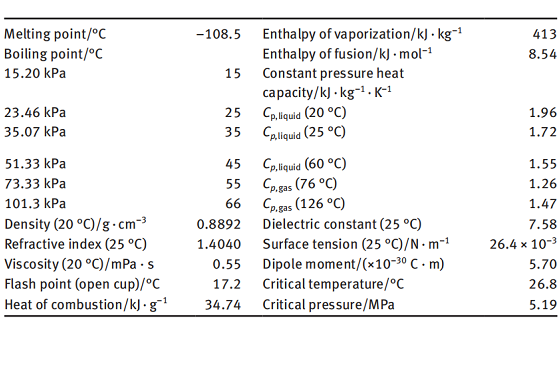
Tetrahydrofuran is a colorless and transparent liquid with an ether-like odor. It is a volatile liquid having a boiling point of 66 °C under atmospheric pressure. It is miscible with water and common organic solvents such as alcohol, ether, ester, ketone, aliphatic hydrocarbon, aromatic hydrocarbon, and halogenated hydrocarbon. Many polymer materials can be dissolved in tetrahydrofuran. It forms an azeotrope with water, having an azeotropic point of 64 °C under atmospheric pressure and a water content of 5.6% (mass fraction) in the azeotrope. As the pressure is increased to 0.8 MPa, the azeotropic point is changed to 106 °C, and the water content in the azeotrope is increased to about 9%. Using this property in industry, an anhydrous product of tetrahydrofuran can be obtained by changing the pressure in the distillations to get rid of its moisture content.
Safety
Tetrahydrofuran is a first-grade flammable product. It can be mixed with air to produce an explosive mixture with an explosion limit of 1.8% to 11.8%. When tetrahydrofuran is in contact with air, it is easy to oxidize with oxygen in the air to form peroxide. Therefore, air should be isolated during the production, storage, transportation, and processing processes, and the sealed container should be filled with nitrogen gas. The antioxidant 2,6-di-tert-butyl-4-methylphenol of 100–200 mg kg–1 is often added to tetrahydrofuran as a stabilizer.
Reaction profile
When tetrahydrofuran is oxidized with nitric acid, succinic acid is formed. Under the catalysis of alumina, tetrahydrofuran is reacted with ammonia at a temperature of 300 to 400 °C to obtain pyrrolidine. Tetrahydrofuran reacts with hydrogen sulfide to form tetrahydrothiophene. In the presence of zinc chloride, it reacts with an acid or an acid chloride to easily open the ring and form BDO or 1,4-dihalide. Chlorination of tetrahydrofuran at room temperature under light results in the formation of 2,3-dichlorotetrahydrofuran. Tetrahydrofuran is dehydrated at 270 °C to form butadiene using acid phosphate as a catalyst. Tetrahydrofuran is hydrolyzed and ring-opened to form a polyether diol in the presence of a strongly acidic catalyst.
Uses
The primary use of tetrahydrofuran is ring-opening polymerization to produce polytetrahydrofuran, which is used as a raw material for the production of polyurethane elastomers, polyurethane elastic fibers, and polyurethane adhesives. Compared with other elastic materials, the material prepared from polytetrahydrofuran has excellent stability against hydrolysis, gas permeability, and wear resistance. It exhibits good elasticity, flexibility, and impact resistance at low temperatures. It has unique and broad application prospects in textiles, pipes, chemicals, medical equipment, etc.
Tetrahydrofuran is an organic solvent with excellent performance. It has a fast dissolution rate and good penetration and diffusion properties on the surface and inside of the resin; therefore, it has been widely used as a solvent for resins. It can dissolve all resins except polyethylene, polypropylene, and fluoro resin, especially for polyvinyl chloride, polyvinylidene chloride, and its copolymers, and results in a low-viscosity solution so that it can be used for polyvinyl chloride pipe processing, manufacture of surface coatings, adhesives, and films as well as inks, extractants, and artificial leather surface treatments.
Tetrahydrofuran is also used to clean polymerization reactors for polyvinyl chloride production. Moreover, it is widely used as a solvent in the Grignard, polymerization, and LiAlH4 reduction reactions. Tetrahydrofuran is also used as an intermediate in organic synthesis and can be applied to synthesize many critical fine chemicals such as tetrahydrothiophene, tetrahydrothiophene, 1,4-dichlorobutane, and pyrrolidine. It is used as a raw material for producing pentoxyverine in the pharmaceutical field.
You may like
Related articles And Qustion
See also
Lastest Price from Tetrahydrofuran manufacturers

US $0.00/kg2025-06-18
- CAS:
- 109-99-9
- Min. Order:
- 1000kg
- Purity:
- i
- Supply Ability:
- 80MT

US $10.00/KG2025-04-21
- CAS:
- 109-99-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt