Canagliflozin hemihydrate: Application, Synthesis, Toxicity and safety
General description
Canagliflozin hemihydrate (Invokana) is a C-glucoside with a thiophene ring that is an orally available inhibitor of sodium-glucose transporter 2 (SGLT2) with antihyperglycemic activity. Canagliflozin is also able to reduce body weight and has a low risk for hypoglycemia. It is a hydrate that is the hemihydrate form of canagliflozin. Used for treatment of type II diabetes via inhibition of sodium-glucose transport protein subtype 2. It has a role as a hypoglycemic agent and a sodium-glucose transport protein subtype 2 inhibitor. It contains a canagliflozin[1].
Application and Pharmacology
Canagliflozin hemihydrate (brand name Invokana) is a medication used to treat type 2 diabetes. It is a sodium-glucose co-transporter 2 (SGLT2) inhibitor that works by preventing the kidneys from reabsorbing glucose into the bloodstream, leading to increased glucose excretion in the urine and lower blood glucose levels. Canagliflozin hemihydrate is taken orally and is usually prescribed as part of a comprehensive diabetes management plan that includes diet, exercise, and other medications. It is important to note that canagliflozin hemihydrate is not recommended for people with type 1 diabetes or diabetic ketoacidosis. It may also not be suitable for people with certain medical conditions or those taking certain medications. As with all medications, it is important to discuss the risks and benefits of canagliflozin hemihydrate with a healthcare professional before starting treatment.
Synthesis
The synthesis of Canagliflozin hemihydrate involves several steps, and there are different synthetic pathways that can be used to produce the drug. Here is a general overview of one possible synthetic pathway:The first step involves the synthesis of a key intermediate compound, (2S,3R,4R,5S,6R)-2-(3,4-dimethoxyphenyl)-6-(4-methylbenzyl)-3,4,5-trihydroxyhexanal. This intermediate is produced by reacting 4-methylbenzaldehyde with D-mannitol in the presence of a catalyst, followed by a series of additional reactions to form the desired product. The next step involves the reaction of the intermediate compound with (2S,3R,4R,5S,6R)-2-(3,4-dimethoxyphenyl)-6-(4-fluorophenyl)-3,4,5-trihydroxyhexane-1,2,5-triol. This reaction results in the formation of canagliflozin, the active ingredient in the drug. Canagliflozin is then reacted with a suitable acid to form a salt, which can be purified and dried to form the final product, it is important to note that the specific details of the synthetic pathway may vary depending on the manufacturer and the particular process used.
Toxicity and safety
Canagliflozin hemihydrate has been extensively studied for its safety and efficacy in treating type 2 diabetes. However, like all medications, it is not without risks and potential side effects. The most common side effects of canagliflozin hemihydrate include urinary tract infections, genital yeast infections, and increased urination. Other possible side effects may include low blood pressure, dizziness, and an increased risk of bone fractures. There have also been some concerns raised about potential cardiovascular risks associated with canagliflozin hemihydrate, particularly in patients with pre-existing cardiovascular disease. The FDA has issued warnings regarding this potential risk and recommends that patients with cardiovascular disease be closely monitored while taking canagliflozin hemihydrate. Additionally, canagliflozin hemihydrate may increase the risk of developing diabetic ketoacidosis, a serious complication of diabetes that can lead to coma or even death. Therefore, it is important that patients taking this medication are aware of the signs and symptoms of diabetic ketoacidosis and seek medical attention if they experience any of these symptoms.
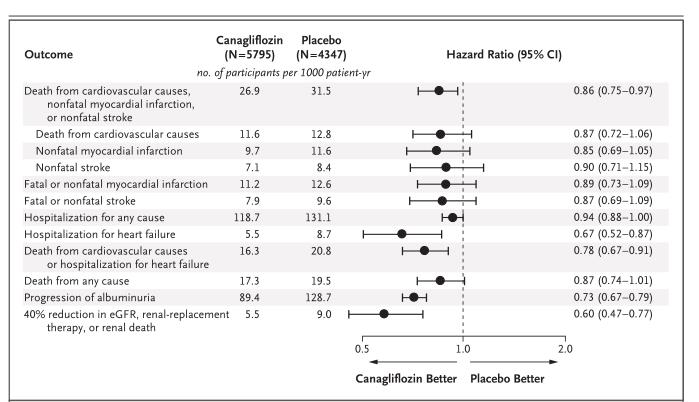 Figure 1 Effects of Canagliflozin on Cardiovascular, Renal, Hospitalization, and Death Events in the Integrated CANVAS Program.
Figure 1 Effects of Canagliflozin on Cardiovascular, Renal, Hospitalization, and Death Events in the Integrated CANVAS Program.
Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. The mean age of the participants was 63.3 years, 35.8% were women, the mean duration of diabetes was 13.5 years, and 65.6% had a history of cardiovascular disease. The rate of the primary outcome was lower with canagliflozin than with placebo (occurring in 26.9 vs. 31.5 participants per 1000 patient-years; hazard ratio, 0.86; 95% confidence interval [CI], 0.75 to 0.97; P<0.001 for noninferiority; P = 0.02 for superiority). Although on the basis of the prespecified hypothesis testing sequence the renal outcomes are not viewed as statistically significant, the results showed a possible benefit of canagliflozin with respect to the progression of albuminuria (hazard ratio, 0.73; 95% CI, 0.67 to 0.79) and the composite outcome of a sustained 40% reduction in the estimated glomerular filtration rate, the need for renal-replacement therapy, or death from renal causes (hazard ratio, 0.60; 95% CI, 0.47 to 0.77). Adverse reactions were consistent with the previously reported risks associated with canagliflozin except for an increased risk of amputation (6.3 vs. 3.4 participants per 1000 patient-years; hazard ratio, 1.97; 95% CI, 1.41 to 2.75); amputations were primarily at the level of the toe or metatarsal. Overall, canagliflozin hemihydrate is considered a safe and effective medication for the treatment of type 2 diabetes when used appropriately and under the guidance of a healthcare professional. However, it is important that patients are aware of the potential risks and side effects of the medication and report any concerns to their doctor or pharmacist.
Reference
1. Neal B., Perkovic V. & Matthews D. R., "Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes," N Engl J Med, Vol.377, No.21(2017), p.2099.
You may like
Related articles And Qustion
Lastest Price from Canagliflozin heMihydrate manufacturers

US $0.00-0.00/kg2025-05-12
- CAS:
- 928672-86-0
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons

US $10.00/KG2025-04-21
- CAS:
- 928672-86-0
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt