Navigating Blood Sugar Control: The Role of Canagliflozin Hemihydrate in Diabetes Care
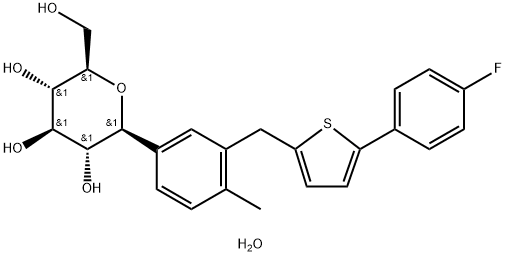
Canagliflozin hemihydrate is a white to off white powder, soluble in many organic solvents (ethanol, methanol, tetrahydrofuran, acetone) but insoluble in aqueous media. The log P of the drug substance is 3.44 at 20℃ and pH=7. There is no pKa in the physiological pH range.

Uses
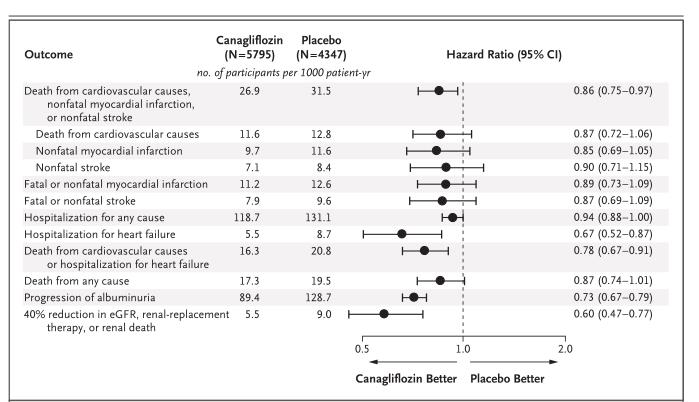
Canagliflozin hemihydrate is indicated to be used with diet and exercise to lower blood sugar in adults with type 2 diabetes; to reduce the risk of major heart-related events such as heart attack, stroke, or death in people with type 2 diabetes who have known heart disease; and to reduce the risk of end-stage kidney disease, worsening of kidney function, heart-related death, and being hospitalized for heart failure in certain people with type 2 diabetes and diabetic kidney disease.
Mechanism of Action
Canagliflozin hemihydrateis an inhibitor of sodium-glucose co-transporter 2 (SGLT2). SGLT2, expressed in the proximal renal tubules, is responsible for the majority of the reabsorption of filtered glucose from the tubular lumen. Patients with diabetes have been shown to have elevated renal glucose reabsorption which may contribute to persistent elevated blood glucose concentrations. Canagliflozin is an orally-active inhibitor of SGLT2. By inhibiting SGLT2, canagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose (RTG), and thereby increases urinary glucose excretion (UGE), lowering elevated plasma glucose concentrations by an insulin-independent mechanism in patients with type 2 diabetes. Urinary glucose excretion induced by canagliflozin leads to an osmotic diuresis, which can be associated with caloric loss and reduction in weight (see PHARMACOLOGY - Clinical Studies).
Metabolism
O-glucuronidation is the major metabolic elimination pathway for canagliflozin, which is mainly glucuronidated by UGT1A9 and UGT2B4 to two inactive O-glucuronide metabolites. CYP3A4-mediated (oxidative) metabolism of canagliflozin is minimal (approximately 7%) in humans.
Genotoxicity
Canagliflozin was not mutagenic with or without metabolic activation in the Ames assay. Canagliflozin was genotoxic in the in vitro mouse lymphoma tk assay with but not without metabolic activation. Canagliflozin was not mutagenic or clastogenic in an in vivo oral micronucleus assay in rats and an in vivo oral Comet assay in rats. The overall weight of evidence indicates that canagliflozin is not genotoxic.
Related articles And Qustion
Lastest Price from Canagliflozin heMihydrate manufacturers

US $0.00-0.00/kg2025-05-12
- CAS:
- 928672-86-0
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons

US $10.00/KG2025-04-21
- CAS:
- 928672-86-0
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt